Translate this page into:
An unusual case of isolated right-sided infective endocarditis masquerading as non-resolving pneumonia
Corresponding Author:
Akshyaya Pradhan
Department of Cardiology, King George Medical University, Lucknow, Uttar Pradesh
India
akshyaya33@yahoo.com
| How to cite this article: Pradhan A, Sanganagoudar SP, Vishwakarma P, Sethi R. An unusual case of isolated right-sided infective endocarditis masquerading as non-resolving pneumonia. Natl Med J India 2019;32:144-146 |
Abstract
Right-sided infective endocarditis in non-intravenous drug abusers and non-immunocompromised patients is rare. The diagnosis is difficult as it can present as a respiratory illness leading to delays in diagnosis and development of complications, which can be fatal. The standard Duke criteria may not be adequate for diagnosis. We present a patient with isolated right-sided infective endocarditis mimicking right lower lobe non-resolving pneumonia who did not respond to antitubercular therapy.
Introduction
Right-sided endocarditis is a well-defined clinical entity in patients with a history of intravenous (i.v.) drug use or who have a pacemaker or other intracardiac device. Its clinical presentation and treatment differ from those of left-sided endocarditis and its prognosis is more favourable, as many patients can be cured with medical treatment alone or with extraction of the surgical device in pacemaker endocarditis. However, many times, atypical presentations such as respiratory infection are possible causing delayed diagnosis and treatment. We describe a patient with right- sided endocarditis masquerading as non-resolving pneumonia.
The Case
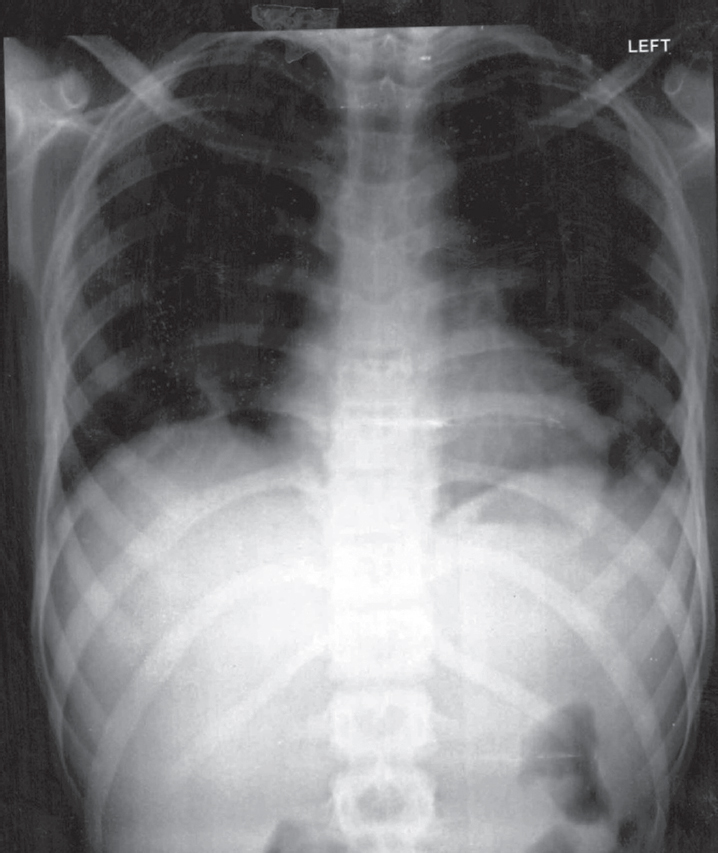
A 16-year-old boy was referred to our centre with a history of febrile illness and a new onset murmur. The patient had a history of febrile illness for 3 months. There was a history of cough with expectoration and chest X-ray revealed right lower zone opacity [Figure - 1]. The patient was evaluated elsewhere and was managed as a case of pneumonia. Initially, the blood cultures were sterile. As the fever was non-responsive, the patient was started on a trial of antitubercular treatment (ATT) empirically. The patient had poor tolerance for ATT and developed liver dysfunction. He had recurrent bouts of fever despite being managed with ATT. Meanwhile, he developed a new-onset murmur for which he was referred to our centre.
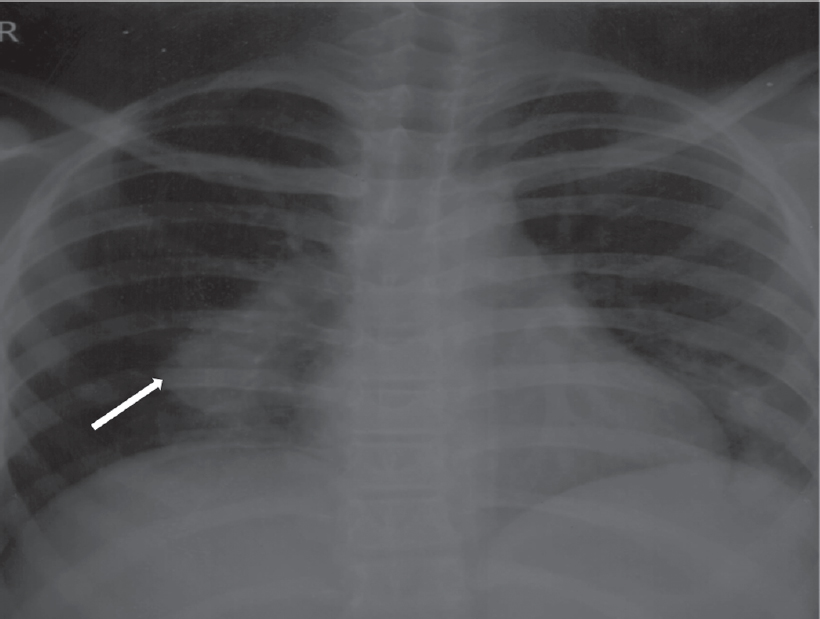 |
| Figure 1: Chest X-ray posterior-anterior view showing right lower lobe consolidation |
On examination, he was febrile but haemodynamically stable. A prominent finding on cardiac auscultation was presence of a grade IV pansystolic murmur on the left sternal border that increased on inspiration.
Electrocardiography was within normal limits with no evidence for any conduction blocks [Figure - 2]. Two-dimensional echocardio- graphy examination showed multiple small mobile intracardiac masses suggestive of vegetations, one each on the anterior tricuspid leaflet (6 mm χ 6 mm) and interventricular septum on the right ventricular side (7 mm χ 7 mm) [Figure - 3]a and [Figure - 3]b. In addition, there was clear evidence of discontinuity of the anterior tricuspid leaflet possibly a perforation [Figure - 4]. Two regurgitant jets were seen on the colour Doppler study, one due to severe tricuspid regurgitation and the other due to perforation of the tricuspid leaflet [Figure - 5]. A second set of blood cultures was positive for Staphylococcus haemolyticus and was sensitive to vancomycin, doxycycline, teicoplanin and gentamicin. The patient was started on i.v. vanco- mycin and oral doxycycline. His fever started regressing from day 2 and he was afebrile thereafter. The patient was continued for 2 weeks with the in-hospital antibiotic regimen while umder watch for renal functions. At discharge, the patient was prescribed oral doxycycline and i.v. gentamicin in a single daily dose for the next 2 weeks. At 2 weeks’ follow-up, he was afebrile and his chest X-ray revealed radiological improvement [Figure - 6].
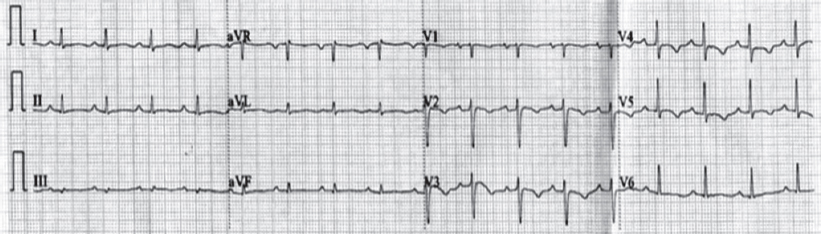 |
| Figure 2: Twelve-lead electrocardiogram showing sinus rhythm |
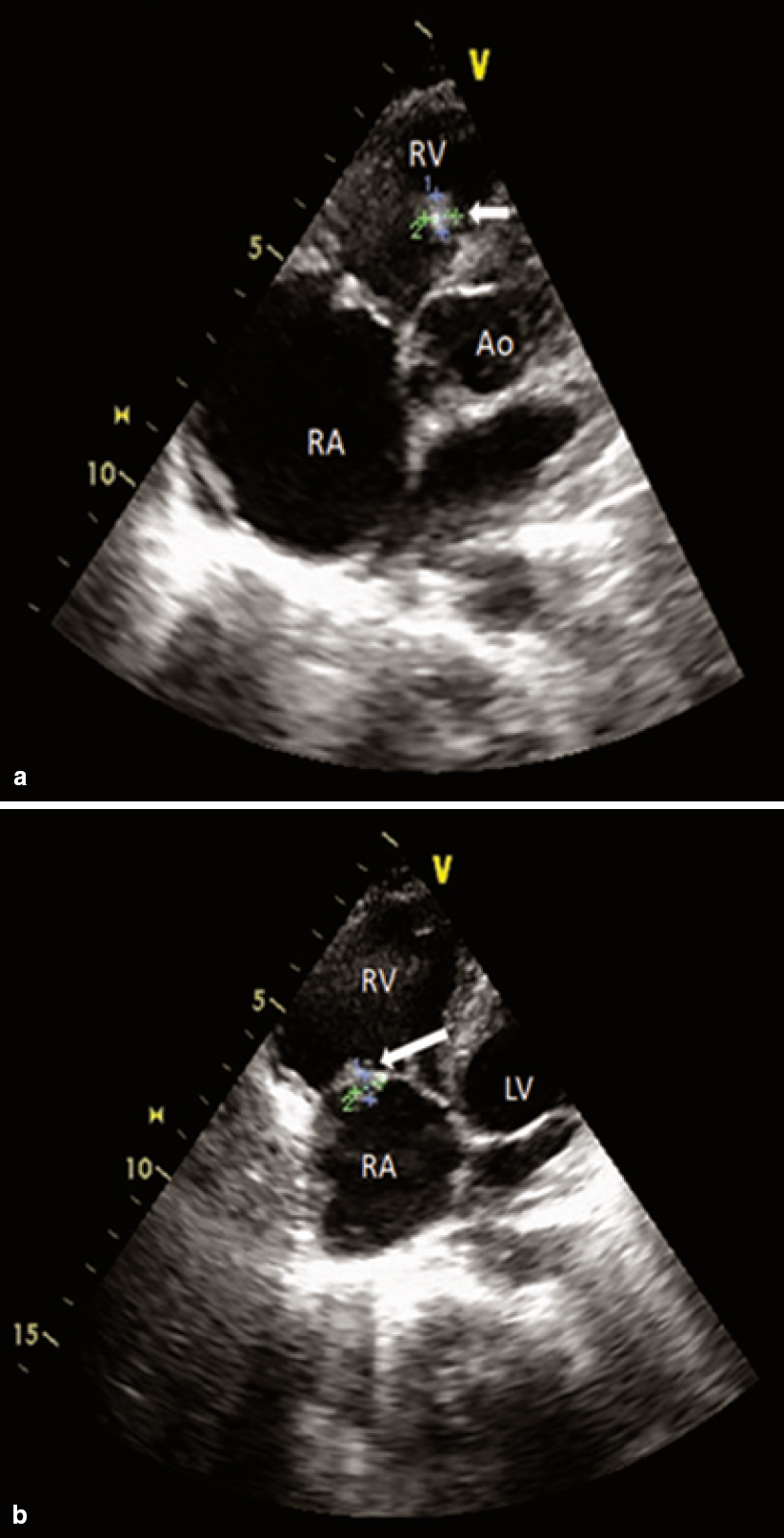 |
| Figure 3: Two-dimensional echocardiogram in multiple planes showing small intracardiac vegetations (white arrows). (a) apical five-chamber view showing mobile vegetation on the right ventricular aspect of interventricular septum; (b) apical four-chamber view showing vegetation on the anterior leaflet of the tricuspid valve and the dilated right atrium. RA right atrium RV right ventricle Ao aorta |
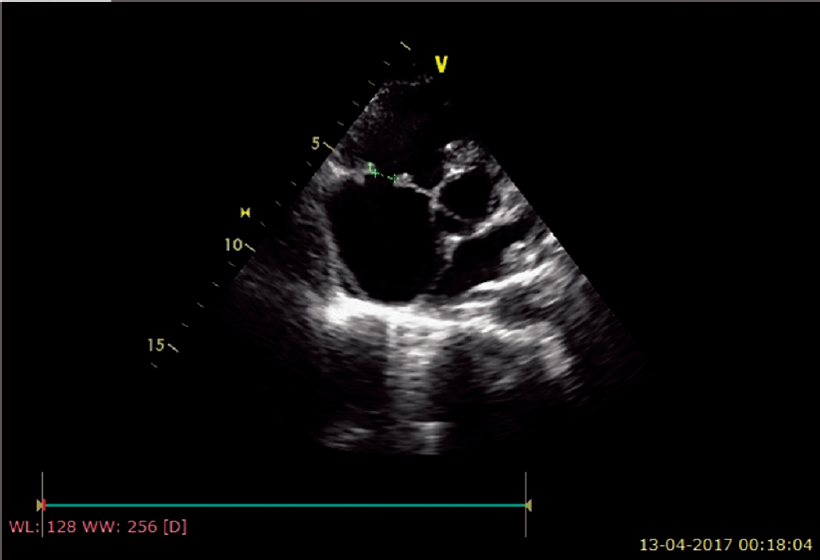 |
| Figure 4: Echocardiogram showing 8 mm perforation of the anterior leaflet of the tricuspid valve |
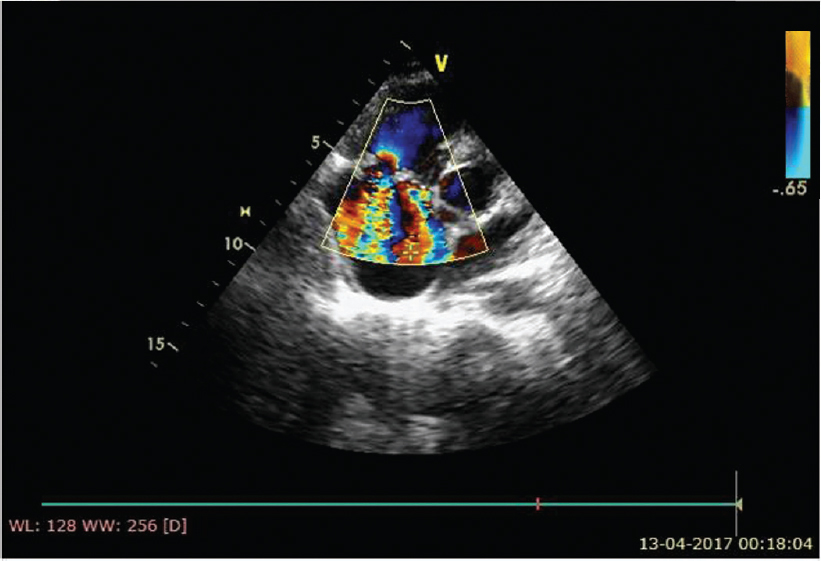 |
| Figure 5: Colour Doppler study showing two separate tricuspid regurgitation jets into the right atrium |
 |
| Figure 6: Follow-up chest X-ray showing radiological improvement |
Discussion
Infective endocarditis has a high in-hospital mortality (15%-30%) and prompt identification as well as initiation of therapy offers an opportunity to change the course of the disease.[1] Right- sided endocarditis is often seen with intracardiac devices or a history of i.v. drug abuse, and many studies have reported these two forms of the disease.[2] Its clinical presentation and treatment differ from those of left-sided endocarditis and its prognosis is more favourable, as many patients can be cured with medical treatment alone or with surgical device extraction in case of pacemaker endocarditis.[1] However, the appearance of right-sided endocarditis in a patient with neither of these two antecedents has rarely been reported, probably because of the low incidence of disease in such patients. The manifesting symptoms of right-sided endocarditis are similar to those of respiratory infection, making a wrong diagnosis likely. In addition, because the clinical condition improves with antibiotic treatment, many cases of right-sided endocarditis remain undiagnosed.
In general, isolated right-sided endocarditis in non-i.v. drug abusers without a pacemaker occurs in young patients.[3] The presence ofpredisposing factors (immunosuppression, renal failure or cancer) is not infrequent. In most instances, the port of entry of the infection can be identified. The most frequent port is through an intravascular catheter, the possibility of developing endocarditis because of a central venous catheter was observed previously.[4] Vascular catheters are the main source of bacteraemias, especially due to infections caused by staphylococci.[5] Delays in the diagnosis are the result, in part, of the fact that the Duke criteria are not appropriate for patients with right-sided endocarditis and according to many the criteria need to be modified.[6] Chest X-ray will frequently reveal septic emboli and pleural effusion. Because respiratory signs and symptoms predominate, patients are often misdiagnosed as having pneumonia. The combination of pulmonary infiltrates and renal disease may also lead to a suspicion of vasculitis.[7] Right-sided endocarditis should be suspected in the presence of the so-called ‘tricuspid syndrome’: recurrent respiratory events, anaemia and microscopic haematuria. The appearance of renal alterations in patients with infective endocarditis is frequent and these alterations can manifest as a variety of complications of varying clinical significance. The most common renal manifestation is haematuria or proteinuria and the most frequent pathological finding is glomerulonephritis, which is usually proliferative and diffuse or vasculitis.
Vegetations are a constant finding. Moreover, these formations were large as a result of the lower pressure exerted by the right heart chambers, which allows vegetations to grow larger than on the left side. A study has associated large, mobile vegetations with a high incidence of embolism.[8]
With respect to outpatient antibiotic therapy, patients whose fever responds do not have any complications and who are expected to be drug compliant can be given outpatient therapy after 2 weeks of inpatient therapy.[1],[7]
Conflicts of interest. None declared
| 1. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36: 3075-128. [Google Scholar] |
| 2. | Moss R, Munt B. Injection drug use and right sided endocarditis. Heart 2003;89: 577-81. [Google Scholar] |
| 3. | Naidoo DP. Right-sided endocarditis in the non-drug addict. Postgrad Med J 1993;69:615-20. [Google Scholar] |
| 4. | Tsao MM, Katz D. Central venous catheter-induced endocarditis: Human correlate of the animal experimental model of endocarditis. Rev Infect Dis 1984;6:783-90. [Google Scholar] |
| 5. | Terpenning MS, Buggy BP, Kauffman CA. Hospital-acquired infective endocarditis. Arch Intern Med 1988;148:1601-3. [Google Scholar] |
| 6. | Robbins MJ, Frater RW, Soeiro R, Frishman WH, Strom JA. Influence of vegetation size on clinical outcome of right-sided infective endocarditis. Am J Med 1986;80: 165-71. [Google Scholar] |
| 7. | Karchmer AW. Infective endocarditis. In: Bonow RO, Mann DM, Zipes DP, Lippy P, Braumwald E, (eds). Braunwald’s heart disease—a textbook of cardivascular medicine. Phildelphia:Elsevier; 2012:1540-61. [Google Scholar] |
| 8. | Tischler MD, Vaitkus PT. The ability of vegetation size on echocardiography to predict clinical complications: A meta-analysis. J Am Soc Echocardiogr 1997;10: 562-8. [Google Scholar] |
Fulltext Views
1,255
PDF downloads
256




