Translate this page into:
Thyroid dysfunction in patients on antiretroviral therapy: A perspective from southern India
2 Department of Biostatistics, St John’s Medical College, Bengaluru 560034, Karnataka, India
3 Department of Endocrinology, St John’s Medical College, Bengaluru 560034, Karnataka, India
Corresponding Author:
Jyothi M Idiculla
Department Of Medicine, St John’s Medical College, Bengaluru 560034, Karnataka
India
jyothi.i@stjohns.in
| How to cite this article: Sebastian SA, Sumithra S, Kurian J, Mathew V, Idiculla JM. Thyroid dysfunction in patients on antiretroviral therapy: A perspective from southern India. Natl Med J India 2018;31:136-139 |
Abstract
Background. Thyroid dysfunction in patients with human retroviral infection has been reported but the prevalence of thyroid function abnormalities in patients on highly active antiretroviral therapy (HAART) has not been studied. We aimed to assess the prevalence of thyroid dysfunction and autoimmunity (antithyroid peroxidase auto-antibodies [TPO-Ab]) in patients on first-line HAART, identify risk factors for thyroid dysfunction and determine any association of thyroid dysfunction with HAART.Methods. We screened and enrolled consecutive patients from the outpatient department if they were (i) diagnosed with HIV infection (enzyme-linked immunosorbent assay); (ii) aged more than 18 years; (iii) on HAART for 1 year or more; and (iv) clinically stable with no evidence of any acute illness in the past 2 months. We excluded patients who were on drugs that affect thyroid function. Thyroid function tests and CD4 counts were done.
Results. A total of 159 patients on firstline HAART were included in the study. Their mean (SD) age was 43.3 (10) years and duration of HAART was 44.4 (33.54) months. The mean CD4 count was 502.8 (274.45). Forty-seven patients (29.6%) had thyroid dysfunction. TPO-Ab positivity was noted in 6 patients. No association was seen between thyroid dysfunction and any type of regimen or drug. There was a significant negative correlation between CD4 counts and thyroid-stimulating harmone (TSH) suggesting that thyroid dysfunction may be more prevalent when immunity is low.
Conclusions. There is a high prevalence of thyroid dysfunction, predominantly subclinical hypothyroidism, in patients on HAART. Thyroid autoimmunity is low in this subset of patients. Lower immunity is associated with higher TSH levels. Larger longitudinal studies are required to determine the course of hypothyroidism in patients on HAART.
Introduction
The association between human immunodeficiency virus (HIV) infection and endocrine abnormalities is well documented in the literature. Before the initiation of highly active antiretroviral therapy (HAART), opportunistic infections were the primary culprit for thyroid dysfunction. However, the effects of HIV on the thyroid gland following HAART are still under scrutiny. In the post-HAART era, it has been suggested that autoimmunity, non-thyroidal illness, HAART drugs and immune reconstitution inflammatory syndrome (IRIS) as a consequence of therapy may all contribute to thyroid dysfunction.[1]
Thyroid dysfunction in patients with HIV has been observed to range from isolated low free T4 (fT4) to overt hypothyroidism.[1] A study from northern India found the prevalence of thyroid dysfunction in the HIV-infected population to be as high as 75.5%.[2] A more recent study from the same centre reported a considerably lower prevalence of 25%.[3] There have been conflicting reports of the association between HAART and thyroid dysfunction.[4],[5] Only a few studies from India have explored thyroid dysfunction in HIV-infected patients on therapy.[2],[3],[6],[7],[8],[9] Moreover, most of the Indian data do not specifically investigate the effect of antiretroviral drugs on thyroid function in the adult population. The conflicting findings and the paucity of data particularly from the southern parts of India prompted this study. Recommendations for thyroid screening for people living with HIV (PLHIV) are much needed particularly in resource-poor settings with a high prevalence of HIV. We hope these data would contribute towards the formulation of guidelines. We aimed to (i) determine the prevalence and describe the pattern of thyroid dysfunction in PLHIV on HAART; (ii) identify risk factors for thyroid dysfunction including the duration and nature of HAART; and (iii) determine the prevalence of thyroid autoimmunity in this subset of PLHIV.
Methods
Study participants
This cross-sectional study was done at St John’s Medical College Hospital, Bengaluru. Consecutive patients were enrolled from the outpatient department if they were (i) diagnosed with HIV infection (enzyme-linked immunosorbent assay); (ii) aged more than 18 years; (iii) on HAART for 1 year or more ; and (iv) clinically stable with no evidence of any acute illness. Patients were excluded if they had any acute illness in the preceding 2 months or if they were on medications that affect thyroid function.
Sample size and statistical methods
Estimation of the sample size was based on the study by Beltran et al.[5] Considering the prevalence of thyroid dysfunction to be 16%, for a 6% precision and 95% confidence interval, the minimum number of participants required for the study was estimated to be 143.
Descriptive statistics were reported using mean (SD) or median with interquartile range (IQR) for continuous variables; numbers and percentages were used for categorical variables. The correlation coefficient was computed to determine the association of thyroid-stimulating hormone (TSH) with CD4 counts, duration of HAART and other variables. The partial correlation coefficient was estimated to determine the relationship between TSH and CD4 counts adjusted for age and duration of HAART. The differences in median TSH values in groups of patients on different HAART drugs were determined using the Mann–Whitney U test. A value of p<0.05 was considered statistically significant. All analyses were done using SPSS version 21.0.
Laboratory assays
CD4 counts were estimated using the flowcytometry method (BD FACS Calibur). Thyroid function tests were done at an accredited laboratory attached to the institution. Free T3 (fT3), fT4 and antithyroid peroxidase antibody (TPO-Ab) were estimated by competitive immunoassay using the chemiluminescent technology. TSH was measured using two-site sandwich immunoassay using direct chemiluminescent technology with constant amount of two antibodies. The Access 2 Beckman Coulter was used to perform the assays. The biological reference ranges for the thyroid function tests were: TSH 0.34–4.1 mIU/ml; fT4 0.61–1.12 ng/dl; fT3 2.5– 3.8 pg/ml. The present reference ranges at our institution are different as the machines have since been recalibrated.
- Subclinical hypothyroidism: TSH between 4.1 and 10 mIU/ml with normal fT4 concentration
- Overt hypothyroidism: TSH >10 mIU/L with low fT4 levels
- Subclinical hyperthyroidism: TSH <0.34 mIU/ml with normal fT4 and/or fT3 levels
- Overt hyperthyroidism: TSH <0.34 mIU/ml with elevated fT4 and/or fT3 levels
- Non-thyroidal illness: Low fT3, low fT4, normal or high TSH levels
- Indeterminate thyroid functions: Isolated rise in fT4 or fT3 with normal TSH and TPO-Ab
- Isolated low fT4: Low fT4 level with fT3 and TSH being normal.
The study was approved by the Institutional Ethics Committee (IEC 162/2015).
Results
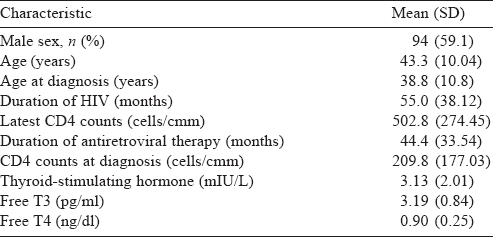
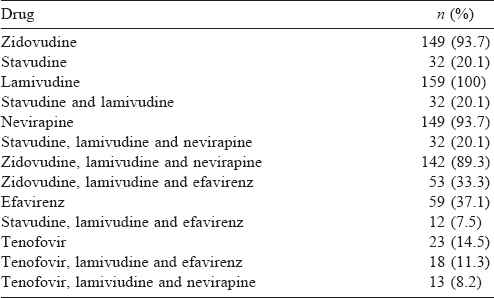
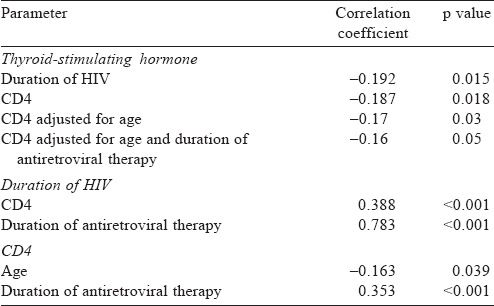
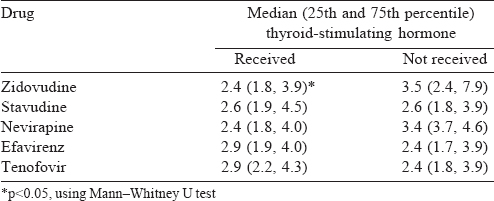
A total of 159 patients were enrolled, of whom 94 were males. The mean (SD) age at diagnosis was 38.8 (10.8) years and the mean (SD) duration of HIV was 55.0 (38.12) months [Table - 1]. The median CD4 count was 460 (IQR 341, 596) and the median TSH was 2.58 (IQR 1.8, 4.0). All patients received lamivudine at some time during treatment [Table - 2]. Thyroid dysfunction was observed in 47 patients. The most commonly observed thyroid function abnormality was subclinical hypothyroidism in 29 patients [Table - 3]. Of the 30 patients with hypothyroidism, only 4 had elevated TPO-Ab titres. TSH levels had a negative correlation with duration of ART and CD4 counts. Age had a negative correlation with CD4 counts. Even after adjusting for age and duration of ART, TSH levels had a negative correlation with CD4 [Table - 4]. The median TSH levels in patients receiving a particular drug [Table - 5] were compared to those of patients who had not received the drug. The only statistically significant difference was noted between patients who had and had not received zidovudine. However, it is clinically irrelevant as the median TSH values were within the normal range.

Discussion
A large cross-sectional population survey done in southern India concluded that the prevalence of thyroid dysfunction in adults is 19.6% (with 9.4% subclinical thyroid dysfunction).[11] The prevalence of thyroid dysfunction documented in persons with HIV in India is much higher, i.e. 23% to 75.5%,[2],[3],[6] whereas in our study it was 29.6%. Studies in the West have reported the prevalence of thyroid dysfunction between 10% and 18%.[4],[5],[12] These wide variations in prevalence across studies may be explained by the heterogeneity of the cohort with regard to the duration and stage of illness, receipt of HAART, nature of HAART, associated infections and severity of immunodeficiency. Differences in the prevalence of thyroidal illness between ethnic communities have been reported in the West.[13] Our results would suggest that such a difference may also be present between people of Indian origin and western populations. These differences may be explained by the lower immunity and greater prevalence of iodine deficiency in our population. The delayed initiation of HAART and poor nutrition in our population would contribute towards lower immunity compared to the western cohort.
The most common pattern of thyroid dysfunction is subclinical hypothyroidism, which is consistent across studies. We documented hypothyroidism in 30 (18.9%) patients, of whom only 1 patient had overt disease. In contrast, the prevalence of subclinical hypothyroidism in southern India is <10%.[11],[14] Besides an underactive thyroid, isolated fT4 and indeterminate results of thyroid function tests were also noted in our study. A similar distribution is seen in studies that have categorized the patterns of dysfunction.[1],[5],[12],[15] Isolated low fT4 was as high as 6.8% in the study by Beltran et al.,[5] and was 3.7% in our study. The pathophysiological basis for this has been suggested to be a hypothyroid-like regulation of the pituitary–thyroid axis. The basis of this hypothesis is the elevated 24-hour mean TSH levels and the higher TSH peak response on thyrotropin-releasing hormone stimulation seen in these patients.[16] Assay interference due to HAART agents may be contributory.[1] While isolated fT4 elevation could be the non-thyroidal illness syndrome, isolated fT3 elevation could not be explained or categorized.
In our study, TPO-Ab positivity in patients with hypothyroidism was low, i.e. only 4 of 30 patients. Increased autoimmunity has been observed in early disease, probably due to the effect of the virus on the host tissues.[17],[18] IRIS has also been observed in this context. The 6.2% TPO-Ab positivity in our study was comparable to the Kolkata report of 3.9%.[3] Silva et al. from Brazil,[19] who found 4. 3 % positivity in patients with thyroid dysfunction, did not discard the possibility of autoimmunity in these immunodeficient people, and suggested the need for tissue studies. The lower autoimmune marker status may point to other considerations in the aetiology of thyroid dysfunction in these patients.
Though the role of HAART is questioned by some studies, associations with nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors have been observed. Silva et al.[19] and Beltran et al.[5] suggested a link between the use of stavudine and thyroid dysfunction, as has been described by Zandman- Goddard and Shoenfeld.[17] In the study by Beltran et al., the odds ratio (OR) for hypothyroidism in patients receiving HAART was 2.7.[5] A correlation with NRTI use and elevated TSH has been reported from Austria in a study that enrolled 410 patients.[20]
In their study on 359 patients (the majority were on HAART), Sharma et al. did not find any association with any of the HAART drugs.[3] Bongiovanni et al. in their study on three groups of patients ranging from ART-naïve to those on at least 1 year of ART also found no association between HAART and thyroid dysfunction.[20] Our study showed no association between thyroid dysfunction and the individual ART drugs.
Another observation from our study is the negative association with CD4 count. Even after adjustments for age and duration of ART, higher CD4 counts were associated with lower TSH.[5] In their study of 50 patients, Jain et al. reported such an association with CD4. They suggested that thyroid abnormalities manifest as CD4 counts fall with advancing disease.[8] Beltran et al. also suggest that low CD4 count is associated with the prevalence of subclinical hypothyroidism with an OR of 2.5 in those with CD4 counts <200.[5] Sharma et al., who made similar observations, suggested that patients with low CD4 counts may have higher viral loads.[3] An Indian study on HIV-infected children has reported an inverse correlation between CD4 and TSH, as observed by us.[9] The association may be due to the increased risk of infections or infiltration of the thyroid gland in patients with lower CD4 counts. However, it requires a tissue diagnosis for confirmation.
The main strength of our study is the inclusion of patients with stable clinical parameters, thus excluding those with the sick euthyroid syndrome. The main drawback of our study is the lack of a comparison group who were not on ART. Anti-thyroglobulin antibodies and imaging studies were also not done.
Conclusion
Subclinical hypothyroidism is the most frequently observed thyroid dysfunction, and TPO-Ab positivity in PLHIV is low. Our study, albeit with limitations, suggests that people with HIV on HAART should be screened for thyroid dysfunction. In this context, it is noteworthy that patients with subclinical hypothyroidism, who have symptoms or suffer from cardiovascular disease require treatment.[21] Larger studies are necessary to evaluate the health of PLHIV and mild thyroid dysfunction as well as pharmaco-economics of screening to better inform guidelines.
Conflicts of interest. None declared
| 1. | Hoffmann CJ, Brown TT. Thyroid function abnormalities in HIV-infected patients. Clin Infect Dis 2007;45:488-94. [Google Scholar] |
| 2. | Dev N, Sahoo R, Kulshreshtha B, Gadpayle AK, Sharma SC. Prevalence of thyroid dysfunction and its correlation with CD4 count in newly-diagnosed HIV-positive adults—A cross-sectional study. Int J STD AIDS 2015;26:965-70. [Google Scholar] |
| 3. | Sharma N, Sharma LK, Dutta D, Gadpayle AK, Anand A, Gaurav K, et al. Prevalence and predictors of thyroid dysfunction in patients with HIV infection and acquired immunodeficiency syndrome: An Indian perspective. J Thyroid Res 2015;2015: 517173. [Google Scholar] |
| 4. | Madge S, Smith CJ, Lampe FC, Thomas M, Johnson MA, Youle M, et al. No association between HIV disease and its treatment and thyroid function. HIV Med 2007;8:22-7. [Google Scholar] |
| 5. | Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, El Esper I, et al. Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: A need for screening. Clin Infect Dis 2003;37:579-83. [Google Scholar] |
| 6. | Meena LP, Rai M, Singh SK, Chakravarty J, Singh A, Goel R, et al. Endocrine changes in male HIV patients. J Assoc Physicians India 2011;59:365-6, 371. [Google Scholar] |
| 7. | Andries A, Isaakidis P, Das M, Khan S, Paryani R, Desai C, et al. High rate of hypothyroidism in multidrug-resistant tuberculosis patients co-infected with HIV in Mumbai, India. PLoS One 2013;8:e78313. [Google Scholar] |
| 8. | Jain G, Devpura G, Gupta BS. Abnormalities in the thyroid function tests as surrogate marker of advancing HIV infection in infected adults. J Assoc Physicians India 2009;57:508-10. [Google Scholar] |
| 9. | Thongam S, Keithelakpam S, Singh TY, Singh RL, Singh AM, Ranabir S, et al. Thyroid dysfunction in human immunodeficiency virus-infected children and its correlation with CD4(+) T lymphocyte count. Indian J Endocrinol Metab 2015;19:272-6. [Google Scholar] |
| 10. | 10. Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM (eds). Williams textbook of endocrinology. 13th ed. Pittsburgh:Elsevier; 2015. [Google Scholar] |
| 11. | Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H, et al. High prevalence of undetected thyroid disorders in an iodine sufficient adult South Indian population. J Indian Med Assoc 2009;107:72-7. [Google Scholar] |
| 12. | Grappin M, Piroth L, Verges B, Sgro C, Mack G, Buisson M, et al. Increased prevalence of subclinical hypothyroidism in HIV patients treated with highly active antiretroviral therapy. AIDS 2000;14:1070-2. [Google Scholar] |
| 13. | Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99. [Google Scholar] |
| 14. | Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N, et al. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab 2013;17:647-52. [Google Scholar] |
| 15. | Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore) 2005;84:98-106. [Google Scholar] |
| 16. | Hommes MJ, Romijn JA, Endert E, Adriaanse R, Brabant G, Eeftinck Schattenkerk JK, et al. Hypothyroid-like regulation of the pituitary-thyroid axis in stable human immunodeficiency virus infection. Metabolism 1993;42:556-61. [Google Scholar] |
| 17. | Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev 2002;1:329-37. [Google Scholar] |
| 18. | Massabki PS, Accetturi C, Nishie IA, da Silva NP, Sato EI, Andrade LE, et al. Clinical implications of autoantibodies in HIV infection. AIDS 1997;11:1845-50. [Google Scholar] |
| 19. | Silva GA, Andrade MC, Sugui Dde A, Nunes RF, Pinto JF, Eyer Silva WA, et al. Association between antiretrovirals and thyroid diseases: A cross-sectional study. Arch Endocrinol Metab 2015;59:116-22. [Google Scholar] |
| 20. | Bongiovanni M, Adorni F, Casana M, Tordato F, Tincati C, Cicconi P, et al. Subclinical hypothyroidism in HIV-infected subjects. J Antimicrob Chemother 2006;58:1086-9. [Google Scholar] |
| 21. | Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18:988-1028. [Google Scholar] |
Fulltext Views
1,727
PDF downloads
10,815




