Translate this page into:
Slow parasite clearance, absent K13-propeller gene polymorphisms and adequate artesunate levels among patients with malaria: A pilot study from southern India
2 Department of Clinical Pharmacology, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
3 Department of Transfusion Medicine and Immunohaematolgy, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
4 Centre for Stem Cell Research, Gene Regulation Laboratory, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
5 Department of Medicine-I and Infectious Diseases, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
6 Special Centre for Molecular Medicine, Jawaharlal Nehru University, New Delhi, India
7 Department of Medicine, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
8 Department of Emergency Medicine, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
9 Department of Biostatistics, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu, India
Corresponding Author:
Priscilla Rupali
Department of Medicine-I and Infectious Diseases, Christian Medical College Hospital, Ida Scudder Road, Vellore 632004, Tamil Nadu
India
priscillarupali@yahoo.com
| How to cite this article: Miraclin T A, Mathew BS, Mammen JJ, Ramachandran SV, Kumar S, Bhattacharjee S, Sudarsanam TD, Sathyendra S, Prabhakar Abhilash K P, Jayaseelan V, Rupali P. Slow parasite clearance, absent K13-propeller gene polymorphisms and adequate artesunate levels among patients with malaria: A pilot study from southern India. Natl Med J India 2019;32:200-206 |
Abstract
Background. Artemisinin-based combination therapy (ACT) is widely used in India and many generic preparations are available. Delayed response has been reported, suggesting inadequate response to artesunate (AS) or genotypic resistance. We designed a prospective observational study to assess the therapeutic response, elaborate pharmacokinetics of AS and identify Plasmodium falciparum kelch 13 (pfk13) propeller gene polymorphisms among hospitalized Indian patients with severe malaria.Methods. We collected blood samples from adult patients with severe P. falciparum or mixed (P. falciparum and P. vivax) malaria on ACT. We calculated the parasite clearance (CL) half-life using the Worldwide Antimalarial Resistance Network (WWARN) online parasite clearance estimator (PCE). We used the liquid chromatography tandem mass spectrophoto-metry method for simultaneous quantification of AS and dihydroartemisinin. We genotyped longitudinally archived DNA samples obtained pre-treatment (day 0) to study the point mutations in the pfk13 propeller domain.
Results. A total of 54 patients with malaria were included, with a majority fulfilling the definitions of severe malaria. The median parasite CL slope half-life was estimated to be 6.44 hours (interquartile range 4.79–10.24). AS pharmacokinetics, assessed in 17 patients, were found to be similar in the groups with rapid (<48 hours) and slow CL (>48 hours) of parasites. No known mutations associated with artemisinin resistance in Southeast Asia were observed in our study participants.
Conclusions. Slow parasite CL was seen with a high parasite burden without genotypic evidence of AS resistance. There is a need to standardize definitions of therapeutic efficacy of AS in cases of severe malaria.
Introduction
Severe malaria contributes to considerable morbidity and mortality in tropical developing nations. In the Southeast Asian region, about 1.3 billion people are at risk of malaria in ten countries, which includes India.[1] Artemisinin and its derivatives are novel agents derived from Artemisia annua, which are proven to be superior to quinine in the management of severe falciparum malaria.[2],[3],[4] Artemisinin-based combination therapy (ACT) has been the standard of care for the management of severe malaria.[5] Global trends over 2010–14 show promising results in the elimination of malaria, as the number of confirmed cases in Asia decreased from 2.9 million to 1.3 million.[1] The efforts at elimination have faced a setback following the emergence of resistance.
The first reported resistance to artemisinin was from the ‘Greater Mekong Region’ in 2009,[6] which prompted further research, leading to important discoveries and updated definitions. Confirmed artemisinin resistance is defined as a phenotype of delayed parasite clearance (CL), as measured by the parasite CL slope half-life >5 hours and genotype harbouring mutations in the kelch 13 propeller region.[7] Assessment of parasite survival rates using the ring-stage survival assay has also been made to substitute parasite CL half-life, to improve the robustness of the definitions.[8] These definitions are standardized for patients with uncomplicated malaria, but are yet to be updated for severe malaria.
The National Antimalarial Drug Resistance Monitoring Network Programme was initiated in 2008, with joint inputs from the National Vector Borne Disease Control Programme (NVBDCP)–National Institute of Malaria Research (NIMR) surveillance system to address the continuing threat of drug- resistant malaria in India.[9] Surveillance data from 1793 patients with Plasmodium falciparum (P. falciparum) malaria collected from 25 sentinel sites from India over 1-year identified 17 patients with confirmed treatment failure.[9] About 47% (8 of 17) of treatment failures had prolonged CL times of >48 hours. Age <5 years and decreased dose of artesunate (AS) (<3 mg/kg) were found to be positively associated with treatment failure; and the lower the dose of AS, the higher was the risk of failure.[9]
Parasite CL has been postulated to be slower in patients with severe malaria due to the high parasite burden.[10] Amaratunga et al. studied the parasite CL rate in a cohort of 30 patients with severe malaria in the Pursat province of Cambodia and found the mean parasite CL half-life to be 5.9 hours and the delay correlated with initial parasite density.[11] Other factors that might contribute to delayed CL include inadequate drug levels, resistance to partner drug, immunity profile of the population and improper practices such as monotherapy and use of counterfeit drugs.[8],[12],[13] We analysed, retrospectively, medical records of hospitalized patients with malaria, and found prolonged parasite and fever CL times.[14] Hence, we did a prospective study to assess the parasite CL rates and AS plasma concentration–time profile and sequenced the K-13 propeller region of P. falciparum, in a longitudinal cohort of patients with severe malaria in southern India.
Methods
Study design and participants
During 2012–15, we included 54 consecutive patients, presenting to Christian Medical College, Vellore, Tamil Nadu, India, a tertiary care referral hospital. Participants were recruited if they were aged >18 years, with microscopy-confirmed malaria as either mono-infection with P. falciparum or mixed infection with P. falciparum and P. vivax and with evidence of severe malaria, identified according to the WHO clinical practice guidelines 2015.[5] Pregnant women and patients who refused to give consent were excluded from the study. Among the 54 patients in the main study, 17 consecutive hospitalized patients with severe malaria were recruited to study the plasma concentration of AS and the metabolite, dihydroartemisinin (DHA). We were unable to do this in all patients due to cost constraints.
Primary outcome measure
Rapid clearance: Rapid CL was defined as the absence of parasitaemia and complete fever defervescence after 48 hours of ACT.
Secondary outcome measures
Delayed clearance: Delayed CL was defined as the persistence of asexual stages in the peripheral smear, 48 hours after initiation of ACT.
Fever clearance time: The fever CL time was defined as the time taken for the temperature to normalize (<38 °C) and remain there for at least 24 hours.
Parasite clearance time: The parasite CL time was defined as the time from the start of treatment until the first negative blood smear.
Ethical approval
The study was approved by the institutional research board (IRB no: 8056/9536) and the study procedures were explained to the participants and relatives using an information sheet. All participants gave written informed consent.
Antimalarials
Intravenous AS was purchased as Azunate® from Macleod Pharmaceuticals. Oral doxycycline was purchased as DOXRID® from Ridley Life Sciences Private Limited. The hospitalized study participants received intravenous AS at a dose of 2.4 mg/ kg at 0, 12 and 24 hours followed by 2.4 mg/kg daily till day 7, along with oral doxycycline 100 mg capsules administered twice daily.[5],[15],[16] The AS was reconstituted with 1 ml of 5% sodium bicarbonate solution to form sodium AS which was further dissolved in 5 ml of 0.9% normal saline and infused. Fourteen patients with malaria received oral ACT (artemether and lumefantrine), as they were able to tolerate the medicines orally.
Admission procedures
At admission, a thorough history including travel history was obtained and clinical examination was done by the principal investigator. Blood samples collected at admission were used for evaluating haematological parameters, parasite index (PI), renal and liver function tests and metabolic parameters. Blood samples for malarial parasites were collected 12-hourly till documented CL of asexual stages of parasites, as seen by a negative smear, was obtained. Blood samples were collected at hour 0 from all patients recruited in the study, before administration of antimalarials and were frozen and stored for genotyping and sequencing of the K-13 propeller region.
Microscopy quality control measures and analysis of parasite clearance half-life
The smears obtained using the quantitative buffy coat and fluorescent microscopic technique with acridine orange stain were used to study the presence of asexual stages of parasites on treatment.[17],[18] PI was calculated by counting the number of parasitized red blood cells (RBCs) among 1000 RBCs. Only asexual forms (ring, trophozoites and schizont) were included for calculating the PI. Parasitaemia (parasites/μl) was calculated from the thin blood film as parasitized red cells per 1000 RBCs × haematocrit × 125.6. All smears were reviewed independently by experienced and qualified laboratory technicians in the clinical pathology laboratories.
Plasma concentration measurement of artesunate and dihydroartemisinin
Two ml of venous blood was obtained from 17 consecutive patients for quantification of AS and DHA. DHA is the normal active metabolite of AS after administration and hence we chose to study its concentration. The first sample was collected before administration of the drug. After slow infusion of AS over 5 minutes, samples were obtained at 5, 7, 9, 15, 30, 45, 60, 90, 120 and 240 minutes from the start of the infusion of AS. The collected blood was immediately centrifuged, plasma was separated and analysed.
Artesunate and dihydroartemisinin quantification
The plasma drug concentrations were assessed using liquid chromatography-tandem mass spectrometry.[19] The drug concentrations differ between people of different ethnicities (3260–28 558 ng/ml).[20]
Molecular studies
Longitudinally archived samples frozen over a period of 3 years from the study patients were used for sequencing of the K-13 propeller region after the study was completed. Nested polymerase chain reaction (PCR) was used to amplify the K-13 propeller gene fragment (codon 427 to 727 on chromosome 13 of 3D7 isolate [PF3D7_1343700]) using known primers.[21] The QIAamp DNA Mini kit was used to extract DNA from the archived whole blood samples. The standardized protocol developed by Ariey et al.[21] was used for genotyping and sequencing the single-nucleotide polymorphisms.
The first round of PCR consisting of 250 nM primers (Sigma® lifesciences, USA), blend master mix (Thermo Fisher Scientific Inc.) and 5 μl of DNA in a total volume of 20 μl was run under the following cycling conditions: 95 °C for 15 minutes; then 30 cycles at 95 °C for 30 seconds; 58 °C for 2 minutes; extension at 72 °C for 2 minutes; and final extension at 72 °C for 10 minutes. The second round of PCR was run with a total volume of 25 μl made of 18.75 μl nuclease-free water, 250 nM primer, blend master mix (Thermo Fisher Scientific Inc.) final concentration and 5 μl of DNA under the following cycling conditions: 95 °C for 15 minutes; then 40 cycles at 95 °C for 30 seconds; 60 °C for 1 minute; extension at 72 °C for 1 minute; and final extension at 72 °C for 10 minutes. Polymorphisms at P. falciparum kelch 13 (pfk13) were determined by direct sequencing of amplicons on an ABI 3730 sequencer (Applied Biosystems, USA), and chromatogram sequences were analysed in comparison to the pfk13-propeller sequence region of 3D7 isolate (Plasmo DB reference ID: Pf3D7_13_v3),[22] manually by three independent investigators.
Statistical analysis
The sample size was estimated using the WHO therapeutic efficacy monitoring guidelines (2009).[23] Using an assumption of 5% failure rate, with 5% precision and 95% confidence interval and 10% loss to follow-up, the required number of study participants was 75. Data were entered in Microsoft Excel Spreadsheets and were analysed with SPSS Inc. Version 16 software. The parasite CL slope half-life of the cohort was estimated by the Worldwide Antimalarial Resistance Network-Parasite Clearance Estimator (WWARN-PCE).[24],[25]
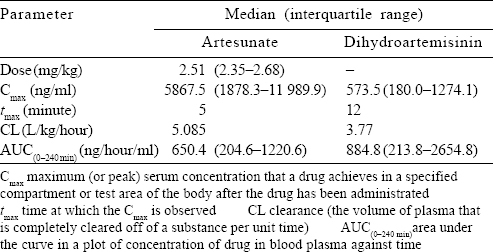
The data on plasma concentration were obtained and the area under the curve (AUC) was calculated for 4 hours’ exposure using the trapezoidal rule. The statistical correlation was obtained through Wilcoxon signed-rank test and Mann–Whitney U tests as applicable. The parameters which were derived included maximal concentration observed (Cmax), terminal elimination half-life (t½), area under the total plasma concentration time curve as a measure of exposure (AUC0–240) and elimination CL. These parameters were tabulated as median with interquartile range. The pharmacokinetic parameters of AS and the metabolite DHA were compared among patients with early CL of parasites within 48 hours and delayed CL of >48 hours, and further analysis was done to ascertain the impact of organ dysfunction on the drug levels.
Results
Baseline characteristics
A total of 54 patients with malaria were enrolled over a period of 3 years (2012–15) from the southern states of Tamil Nadu and Andhra Pradesh. This cohort consisted of hospitalized patients with malaria, where 74% were admitted with severe malaria. Among the patients hospitalized with severe malaria, 17 consecutive patients were enrolled into the study on AS pharmacokinetics. The mean (SD) age of the cohort was 37.5 (14.1) years (interquartile range [IQR] 17–75 years). There was a preponderance of men at 85%, with about 47% of them self-employed as agricultural labourers. Twenty-six per cent of them were skilled professionals, residing in places endemic for malaria during the monsoons, 21% were skilled workers and the remaining 6% were unemployed.
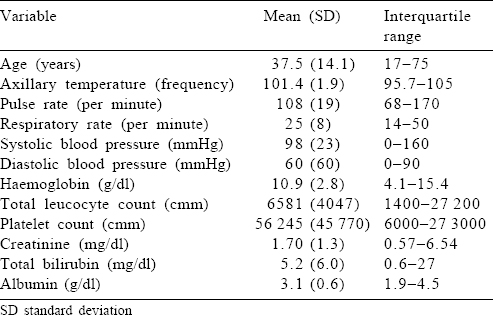
The most common presenting symptom was fever, which was present in all the patients. The median duration of fever before presentation was 7 days (IQR 4–60 days). About 61% reported jaundice (defined by yellowish discolouration of skin, mucous membranes or high-coloured urine). Breathing difficulty or chest tightness at presentation was present in about one-fourth of the patients. Altered sensorium was present in 24%, which included drowsiness, decreased verbalization or irrelevant speech and inadequate response to call or commands. Two patients had seizures at presentation, which were generalized tonic–clonic convulsions requiring antiepileptic therapy. Of the bleeding manifestations, 6 patients reported haematuria, 4 had haematemesis suggestive of upper gastrointestinal (GI) bleed and 2 reported epistaxis. The mean parasite index at admission was 5.51% (IQR 0.1%–48%) and the median parasite CL of the cohort was 0.2% (SD 11.4). All participants received acetaminophen for fever and proton pump inhibitors as prophylaxis for GI bleed. The baseline clinical and laboratory parameters are summarized in [Table - 1].
Therapeutic response
A majority of participants tolerated AS well and showed rapid clinical response in the form of defervescence of fever and improvement in laboratory parameters. The median parasite CL time was estimated to be 36 hours (95% CI 27.0–44.9) and the median fever CL time was estimated to be 24 hours (95% CI 18.6–29.3), indicating high therapeutic efficacy of ACT. The median slope half-life was estimated at 6.44 hours (IQR 4.7–10.2). This median slope half-life was generated from the WWARN-PCE. Almost one-fourth of the patients still had detectable gametocytaemia in the peripheral smear after 48 hours on ACT. There were two deaths, both of them within 3 hours of admission into the emergency department. No adverse events were reported.
Pharmacokinetics of artesunate and dihydroartemisinin (n=16) The pharmacokinetic study was conducted after 48 hours of admission to hospital. Seventeen patients consented for the study procedures. However, 1 patient was excluded from the analysis as the study could not be completed in the patient.
Post-administration of AS, the plasma concentration was achieved in about 5 minutes. The pharmacokinetic parameters are summarized in [Table - 2] and [Figure - 1] and [Figure - 2].
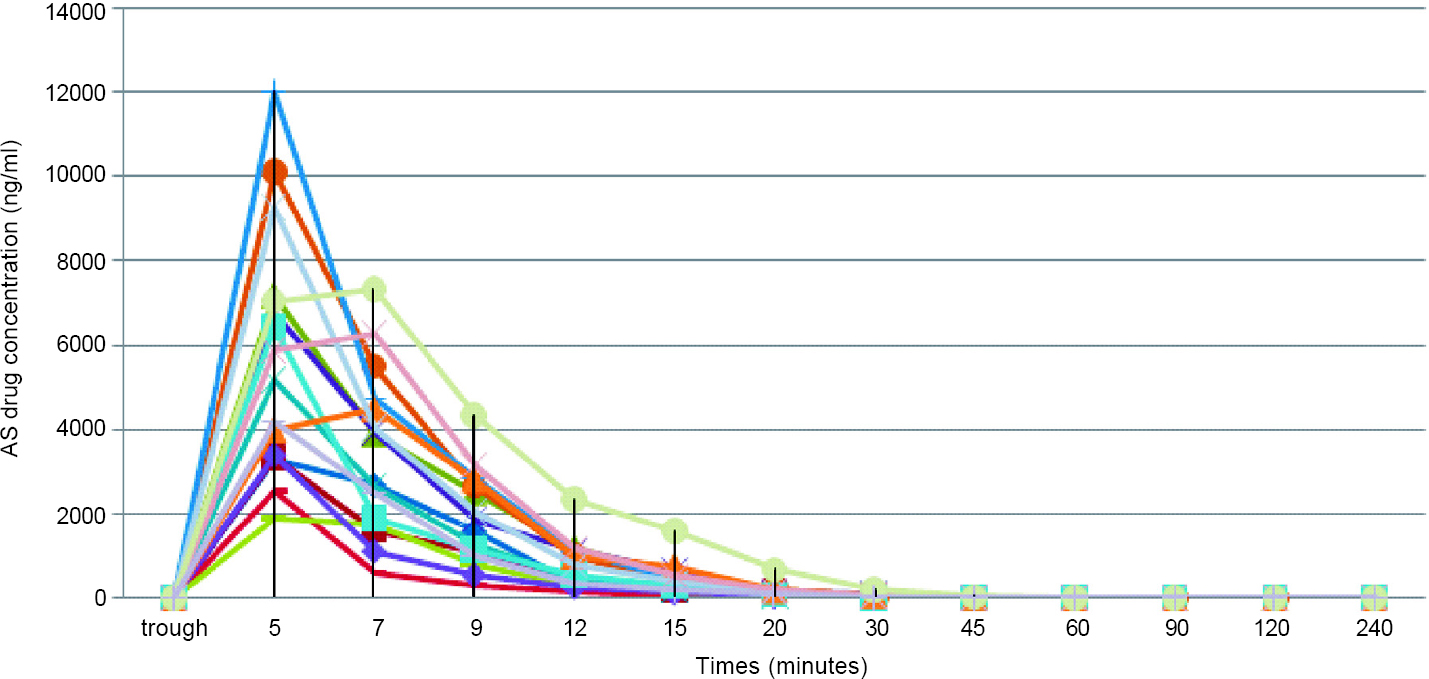 |
| Figure 1: Pharmacokinetics of artesunate (AS) |
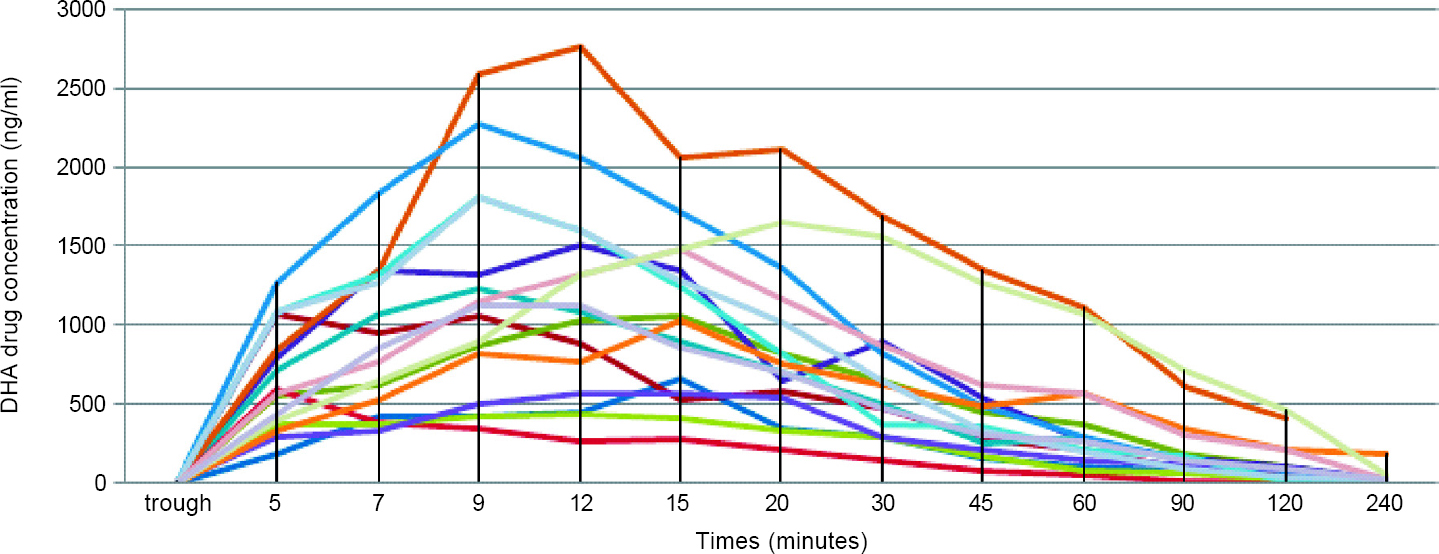 |
| Figure 2: Pharmacokinetics of dihydroartemisinin (DHA)––active metabolite of artesunate in the human body after intravenous administration |
Comparison of parasitological and pharmacokinetic variables
The pharmacokinetic parameters were compared between the two groups of patients who cleared trophozoites rapidly (up to 48 hours) and those who took longer (>48 hours) and the drug exposure variables were not found to be statistically different [Table - 3].
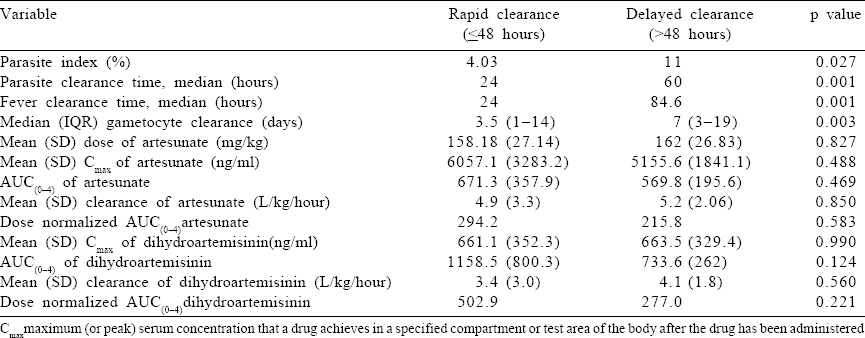
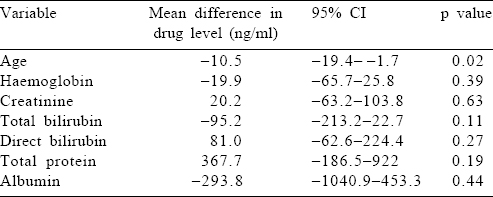
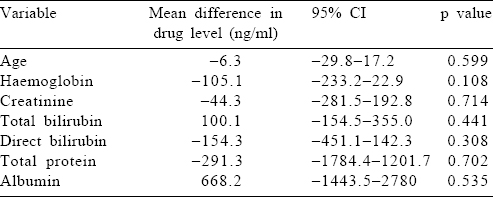
Factors affecting the area under the curve of artesunate and dihydroartemisinin
We studied the effect of hyperparasitaemia, hypoalbuminaemia, and renal and hepatic dysfunction on the metabolism of AS by correlating these host parameters to the AUC(0–240 minutes) of AS and DHA, the active metabolites of AS [Table - 4] and [Table - 5]. We did not find any factor that significantly altered the drug levels. However, it was interesting that a unit increase in age, serum creatinine and bilirubin (total and direct) led to a drop in the drug levels (AS and DHA). However, this drop was not statistically significant.
K13-propeller gene amplification and sequencing analysis
The full-length K-13 propeller genes on chromosome 13 from codon 427 to 727 were sequenced from 53 isolates. The primary PCR was positive in 42 isolates and was sequenced. The primary PCR failed to amplify the Pfkelch13 in 11 isolates. This was probably secondary to the low parasite density in these samples. Among these 42 sequenced isolates, all of them had pfk13 loci coding for the propeller domain identical to the reference strain. There were no mutations or single-nucleotide polymorphisms identified in the sequenced isolates.
Discussion
Our pilot study done in southern India correlated AS drug levels with clinical response in patients with severe malaria. We also aimed to investigate the therapeutic response of ACT in the management of severe malaria and the pharmacokinetics of AS in severe malaria and ascertain the presence of artemisinin resistance mutations in the pfk13-encoded propeller domain. The preponderance of men in our cohort might be explained by the health-seeking behaviour of the rural population, where they can easily access the healthcare facility without social inhibition. Evidence suggests that given equal exposure, adult men and women are equally vulnerable to severe malarial infection, except for pregnant women who are at a higher risk of severe malaria in most endemic areas.[26],[27] Men have a higher occupational risk of contracting malaria than women, particularly if men work in mines, fields or forests at peak biting times, or migrate to areas of high endemicity for work.[26] The presentation of severe malaria in our patients was similar to the experience worldwide.[28],[29]
The majority of participants responded rapidly to ACT, as evidenced by rapid defervescence of fever and CL of asexual parasites from the peripheral blood in 48 hours. This adds to the existing evidence that ACT can be successfully used as first-line therapy in the management of severe malaria even in South Asia.[2],[3],[5] However, the parasite CL half-life was found to be 6.44 hours, which is higher than the cut-off of 5 hours attributed to the definition of ‘partial artemisinin resistance’ according to the existing WHO guidelines.[30] There is paucity of data with regard to parasite CL in severe malaria from areas with a high parasite density. A pilot study by Amaratunga et al. on 30 patients with severe malaria showed delayed parasite CL rates among patients with complicated and uncomplicated malaria, and currently, it is unclear whether a high parasite density affects parasite CL rates.[11] Hence, the definitions described by WHO for ‘artemsinin resistance’ though can be applied for uncomplicated falciparum malaria may not be applicable for severe malaria. However, the trend of increased parasite CL half-life in severe malaria treated with artemisinin is worrying, and continued surveillance to ensure appropriate use and treatment response is essential.
Our study also gives insights into the pharmacokinetics of AS in Indian patients with severe malaria. As previously reported, AS achieved high serum concentrations within 5 minutes and was rapidly hydrolysed to its metabolite DHA in 12 minutes, which makes it an ideal antimalarial agent for the management of severe falciparum malaria.[4],[16],[31],[32],[33],[34] The AUC for AS in our study population was 650.4 ng/ml, whereas in the Vietnamese cohort, it was 2980 ng/ml and in the African cohort, it was 727 ng/ml. In a published review detailing clinical pharmacokinetic parameters of AS in malaria, the authors reported[20] that the mean CL of AS was 2–3 L/kg/hour and for DHA was 0.5–1.5 L/kg/hour. In comparison to this, our study population had higher mean rates of CL of 4.95 and 3.41 L/kg/hour, respectively. Davis et al.[34] suggested that higher doses of AS contributed to a better parasiticidal effect; however, which pharmacokinetic parameters could determine a dosing schedule was unclear. Clinical dose finding studies have agreed upon the dose of 2.4 mg/kg, which is currently the standard of care in our population.[2],[3],[20],[34] The question of whether our population requires higher dosing at baseline needs to be answered through further research, taking into account the proportion of treatment failure rates as well as the pharmacogenomics of the drug.
We compared the parasite index at admission and renal and hepatic functions with the mean AUC(0–240) of AS and DHA. The concentrations were found to be lower in patients with higher age, renal dysfunction and hepatic dysfunction, suggesting that there may be a varying volume of distribution in patients with severe malaria. Hence, in view of this difference of drug concentrations in patients with severe malaria, it is possible that a higher dosing regimen is required in older patients and those with considerable organ dysfunction.
Our study did not find any mutation associated with artemisinin resistance in the pfk13-propeller region. Three point mutations, namely C580Y, R539T and G533A, were originally described to be associated with artemisinin resistance.[21] Recently published evidence on surveillance of pfk-13 point mutations in India identified three patients from northeast India, harbouring non-synonymous mutations; however, they did not have ACT treatment failure.[35] Our results are comparable to the surveillance study done by Mishra et al. in 2015,[35] where the Pfk-13 was amplified successfully from all responders; however, it could not be amplified in five samples, which were obtained from non-responders. DNA sequence analysis of Pfk-13 from the 384 clinical isolates showed six point mutations and one deletion. The 11 isolates which we were not able to amplify had a mean parasite density of 0.2%, and all of them responded to therapy.
Surveillance studies from Africa have also shown a lack of these mutations despite frequent and widespread use of these antimalarials and a high parasite biodensity.[36] It is unclear at the moment whether the phenotypic evidence of ACT resistance/ failure should be based on the parasite CL dynamics even in severe malaria or whether these definitions will need to be modified. If parasite CL continues to be delayed with persistent gametocytaemia in the absence of known mutations in the pfk13-propeller region, possible whole-genome sequencing to identify novel mutations in other parts of the parasite genome conferring resistance to ACT would be the need of the hour. Another explanation for the lack of mutations would be the use of the day 0 sample for PCR and sequencing. This might have further led to an under representation of resistant strains.
Limitations
The major limitation of the study is its small sample size. This hospital-based study was conducted in a tertiary care referral centre which caters to two major southern states of India, which have seasonal transmission of malaria. There is a surge in the numbers immediately after the monsoon season, and only patients with severe malaria are referred and hospitalized. Hence, this sample population might not be representative of the community.
Conclusion
Our study shows that ACT is the treatment of choice in severe malaria in India with a reassuring absence of mutations in pfk13- propeller region of P. falciparum. Parasite CL was delayed in patients with severe malaria suggesting the need for standardized definitions for artemisinin resistance in these patients, as the existing WHO definitions are applicable only for uncomplicated malaria. Plasma AS and DHA concentrations were lower in older patients and those with organ dysfunction, hence probably indicating a need for higher doses of AS at shorter intervals in Indian patients with severe malaria.
Conflicts of interest. None declared
| 1. | World Health Organization. World Malaria Report 2015. Available at www.who.int/ malaria/publications/world-malaria-report-2015/report/en/ (accessed on 15 Jan 2017). [Google Scholar] |
| 2. | Dondorp A, Nosten F, Stepniewska K, Day N, White N; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group, et al. Artesunate versus quinine for treatment of severe falciparum malaria: A randomised trial. Lancet 2005;366:717-25. [Google Scholar] |
| 3. | Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet 2010;376: 1647-57. [Google Scholar] |
| 4. | Price RN. Artemisinin drugs: Novel antimalarial agents. Expert Opin Investig Drugs 2000;9:1815-27. [Google Scholar] |
| 5. | World Health Organization. Guidelines for treatment of malaria. 3rd ed. Geneva:World Health Organization; 2015. Available at www.who.int/docs/default-source/documents/publications/gmp/guidelines-for-the-treatment-of-malaria-eng.pdf?sfvrsn= a0138b77_2 (accessed on 15 Jan 2017). [Google Scholar] |
| 6. | Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455-67. [Google Scholar] |
| 7. | World Health Organization. Status report on artemisinin and ACT resistance (September 2015). Geneva:World Health Organization; 2015. Available at www. who. int/malaria/publications/atoz/update-artemisinin-resistance-sep2015/en/ (accessed on 15 Jan 2017). [Google Scholar] |
| 8. | Amaratunga C, Witkowski B, Khim N, Menard D, Fairhurst RM. Artemisinin resistance in Plasmodium falciparum. Lancet Infect Dis 2014;14:449-50. [Google Scholar] |
| 9. | Mishra N, Singh JP, Srivastava B, Arora U, Shah NK, Ghosh SK, et al Monitoring antimalarial drug resistance in India via sentinel sites: Outcomes and risk factors for treatment failure, 2009-2010. Bull World Health Organ 2012;90:895-904. [Google Scholar] |
| 10. | Severe falciparum malaria. World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg 2000;94 (Suppl 1):S1-S90. [Google Scholar] |
| 11. | Amaratunga C, Mao S, Sreng S, Suon S, Fairhurst RM. Slow parasite clearance rates in response to artemether in patients with severe malaria. Lancet Infect Dis 2013;13:113-14. [Google Scholar] |
| 12. | Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis 2015;211:670-9. [Google Scholar] |
| 13. | Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014;371:411-23. [Google Scholar] |
| 14. | Miraclin TA, Matthew A, Rupali P. Decreased response to artemisinin combination therapy in falciparum malaria: A preliminary report from South India. Trop Parasitol 2016;6:85-6. [Google Scholar] |
| 15. | Basco LK, Le Bras J. Activity in vitro of doxycycline against multidrug-resistant Plasmodium falciparum. Trans R Soc Trop Med Hyg 1993;87:469-70. [Google Scholar] |
| 16. | German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet 2008;47:91-102. [Google Scholar] |
| 17. | Lowe BS, Jeffa NK, New L, Pedersen C, Engbaek K, Marsh K, et al. Acridine orange fluorescence techniques as alternatives to traditional Giemsa staining for the diagnosis of malaria in developing countries. Trans R Soc Trop Med Hyg 1996;90:34-6. [Google Scholar] |
| 18. | Nandwani S, Mathur M, Rawat S. Evaluation of the direct acridine orange staining method and Q.B.C. test for diagnosis of malaria in Delhi, India. J Commun Dis 2003;35:279-82. [Google Scholar] |
| 19. | Geditz MC, Heinkele G, Ahmed A, Kremsner PG, Kerb R, Schwab M, et al. LC-MS/MS method for the simultaneous quantification of artesunate and its metabolites dihydroartemisinin and dihydroartemisinin glucuronide in human plasma. Anal Bioanal Chem 2014;406:4299-308. [Google Scholar] |
| 20. | Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L, et al. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J 2011 ;10:263. [Google Scholar] |
| 21. | Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014;505:50-5. [Google Scholar] |
| 22. | Henriques G, Hallett RL, Beshir KB, Gadalla NB, Johnson RE, Burrow R, et al. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis 2014;210:2001-8. [Google Scholar] |
| 23. | World Health Organization. Methods for surveillance of antimalarial drug efficacy. Geneva:World Health Organization; 2009. Available at www.who.int/malaria/ publications/atoz/9789241597531/en/ (accessed on 15 Jan 2017). [Google Scholar] |
| 24. | Parasite Clearance Estimator (PCE) | Worldwide antimalarial resistance network. Available at www.wwarn.org/tools-resources/toolkit/analyse/parasite-clearance-estimator-pce (accessed on 13 Jul 2016). [Google Scholar] |
| 25. | Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: The parasite clearance estimator. Malar J 2011 ;10:339. [Google Scholar] |
| 26. | World Health Organization. Gender, health and malaria. Available at www.who.int/ gender/documents/gender_health_malaria.pdf (accessed on 13 Jul 2016). [Google Scholar] |
| 27. | Reuben R. Women and malaria--Special risks and appropriate control strategy. Soc Sci Med 1993;37:473-80. [Google Scholar] |
| 28. | Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care 2003;7:315-23. [Google Scholar] |
| 29. | Bruneel F, Hocqueloux L, Alberti C, Wolff M, Chevret S, Bédos JP, et al The clinical spectrum of severe imported falciparum malaria in the Intensive Care Unit: Report of 188 cases in adults. Am J Respir Crit Care Med 2003;167:684-9. [Google Scholar] |
| 30. | World Health Organization. Artemisinin and artemisinin-based combination therapy resistance: Status report. Geneva:World Health Organization; 2016. Available at https://apps.who.int/iris/handle/10665/208820 (accessed on 17 Jan 2017). [Google Scholar] |
| 31. | Kremsner PG, Taylor T, Issifou S, Kombila M, Chimalizeni Y, Kawaza K, et al. A simplified intravenous artesunate regimen for severe malaria. J Infect Dis 2012; 205:312-19. [Google Scholar] |
| 32. | Ambroise-Thomas P. Current data on major novel antimalaria drugs: Artemisinin (qinghaosu) derivatives. Bull Acad Natl Med 1999;183:797-80. [Google Scholar] |
| 33. | Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Mayanja-Kizza H, Katabira E, et al. Pharmacokinetics and pharmacodynamics of intravenous artesunate during severe malaria treatment in Ugandan adults. Malar J 2012;11:132. [Google Scholar] |
| 34. | Davis TM, Phuong HL, Ilett KF, Hung NC, Batty KT, Phuong VD, et al. Pharmaco-kinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob Agents Chemother 2001;45:181-6. [Google Scholar] |
| 35. | Mishra N, Prajapati SK, Kaitholia K, Bharti RS, Srivastava B, Phookan S, et al. Surveillance of artemisinin resistance in Plasmodium falciparum in India using the kelch13 molecular marker. Antimicrob Agents Chemother 2015;59:2548-53. [Google Scholar] |
| 36. | Muwanguzi J, Henriques G, Sawa P, Bousema T, Sutherland CJ, Beshir KB, et al. Lack of K13 mutations in Plasmodium falciparum persisting after artemisinin combination therapy treatment of Kenyan children. Malar J 2016;15:36. [Google Scholar] |
Fulltext Views
1,259
PDF downloads
297




