Translate this page into:
An unusual left ventricular outflow tract mass in a patient with systemic lupus erythematosus
2 Department of Cardiology, Max Super Specialty Hospital, New Delhi, India
Corresponding Author:
Manish Bansal
Departments of Cardiology, Cardiothoracic Surgery and Pathology, Medanta - The Medicity, Sector 38, Gurgaon 122001, Haryana
India
manishaiims@hotmail.com
| How to cite this article: Grewal HK, Bansal M, Mehrotra R, Kumar R, Salwan R, Bhan A, Gautam D, Kasliwal RR. An unusual left ventricular outflow tract mass in a patient with systemic lupus erythematosus. Natl Med J India 2020;33:19-21 |
Abstract
A 25-year-old female, with systemic lupus erythematosus and antiphospholipid antibody syndrome, presented with exertional dyspnoea. Echocardiography showed a large (2.0 cm × 1.1 cm), echogenic, heterogeneous mass in the left ventricular outflow tract, under the aortic valve, attached to the ventricular aspect of the anterior mitral leaflet. Tiny flagellar, frond-like structures were seen attached to the surface of the mass. There was mitral regurgitation. These echocardiographic features were suggestive of a papillary fibroelastoma, but the histopathology of the excised mass revealed it to be a thrombus, which was consistent with a diagnosis of non-bacterial thrombotic endocarditis (NBTE). This case represents a rare histopathologically confirmed NBTE presenting as an unusually large mass in the left ventricular outflow tract.Introduction
Non-bacterial thrombotic endocarditis (NBTE) is a rare entity characterized by the formation of sterile intracardiac vegetations, with aortic and mitral valve leaflets being the commonest sites. Connective tissue diseases, especially in the presence of antiphospholipid antibody (APLA) syndrome, and malignancies are the most common underlying causes of NBTE. Although vegetations in NBTE have characteristic echocardiographic appearance, they can present in many atypical forms, leading to diagnostic and therapeutic dilemmas. We report here a histologically confirmed NBTE presenting as an unusually large mass in the left ventricular outflow tract with highly atypical echocardiographic appearance.
The Case
A 25-year-old female presented to us with complaints of effort dyspnoea and fatigue on minimal exertion. She was known to have systemic lupus erythematosus (SLE) with APLA syndrome. She had had recurrent abortions and was on oral anticoagulants. During this presentation, she had no history of fever, palpitations, syncope or any transient focal neurological deficit. Cardiovascular examination revealed a pan-systolic murmur, best audible in the apical area, suggestive of mitral regurgitation (MR). The rest of the examination was unremarkable.
Initial laboratory investigations revealed haemoglobin of 9.8 g/dl, normal white cell count and thrombocytopenia (platelet count was 80 000/cmm). She was on warfarin and her coagulation parameters were abnormal. Liver and renal function tests were normal, and the blood cultures were sterile. She had borderline positive anti-histone antibodies but all the other antinuclear antibody tests were negative.
Echocardiography, initially transthoracic and subsequently transoesophageal, was performed given the presentation with exertional dyspnoea and clinical examination suggestive of considerable MR. It revealed a large (2.0 cm×1.1 cm), echogenic, heterogeneous mass in the left ventricular outflow tract, only under the aortic valve [Figure - 1]. The mass was attached to the ventricular aspect of the anterior mitral leaflet (AML), just near the aorto-mitral junction. Numerous tiny flagellar, frond-like structures were seen attached to the surface of the mass. The mitral valve leaflets were mildly thickened with malcoaptation resulting in moderately severe MR. The left ventricle was mildly dilated, but ejection fraction was preserved. There was no tricuspid regurgitation and inferior vena cava was normal, indicating normal systemic venous pressure.
 |
| Figure 1: Echocardiography showing an echogenic mass (arrows) attached to the ventricular aspect of the anterior mitral leaflet, predominantly located in the left ventricular outflow tract, just under the aortic valve: (A) parasternal long-axis view; (B) parasternal short-axis view; (C) apical four-chamber view; and (D) apical long-axis view AML anterior mitral leaflet AoV aortic valve LA left atrium LV left ventricle |
Although NBTE was the first possible cause because of the background of SLE and APLA, the echocardiographic features were unlike of what have been described for NBTE. The vegetations in NBTE are generally small in size (usually 5–10 mm), sessile, dome-shaped and characteristically occur at the coapting edge of the valve leaflets. Although these can occur on either side of the valves, but are more commonly seen on the atrial aspect of the mitral and tricuspid leaflets and the ventricular aspect of the aortic and pulmonary leaflets. These vegetations usually do not cause any damage to the underlying leaflets and do not alter or impede valve function. Frond-like oscillating structures are also uncommon in NBTE. The echocardiographic features in our case were distinct from these and were suggestive of a papillary fibroelastoma.
The patient underwent further evaluation in view of atypical echocardiographic findings. Her serum inflammatory markers were elevated (erythrocyte sedimentation rate 66 mm in the 1st hour and C-reactive protein [CRP] 59.9 mg/L), but there was no other evidence of infective endocarditis (white cell count was normal; three separate blood cultures were sterile). Cardiac magnetic resonance imaging (MRI) was performed which, though was non-confirmatory, was more in favour of a papillary fibroelastoma.
In view of the persistent diagnostic uncertainty, clinical presentation with exertional dyspnoea with considerable MR and high embolic potential of the mass with the risk of devastating complications, the mass was excised surgically. The morphological features suggested by preoperative echocardiography were confirmed intraoperatively by direct visual inspection. The underlying AML was damaged; hence, mitral valve replacement was done with a 27-mm size bileaflet mechanical prosthesis. The patient had an uneventful recovery. Histopathology examination of the mass showed only a thrombus without any inflammatory cells or tissue components, consistent with a diagnosis of NBTE. Periodic acid–Schiff staining ruled out any fungal endocarditis [Figure - 2]. The underlying mitral valve tissue showed non-specific degenerative changes.
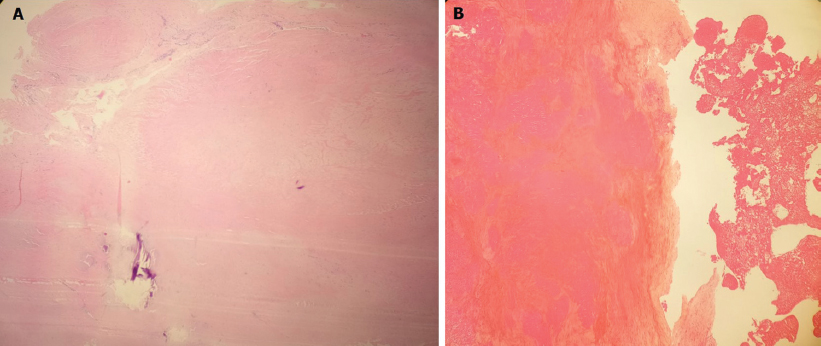 |
| Figure 2: (A) Histopathology of the excised mass showing the only thrombus without any inflammatory cells or tissue components; (B) periodic acid-Schiff staining of the excised mass confirms the absence of any fungal endocarditis |
Discussion
We report a histologically confirmed NBTE presenting as an unusually large mass in the left ventricular outflow tract. Because of the atypical morphology of the mass, echocardiography and cardiac MRI were non-diagnostic, and histopathology could confirm the diagnosis.
NBTE is a rare entity in which small sterile vegetations form on normal heart valves in the absence of bacterial infection in the blood.[1] Although the true incidence of NBTE is not known, it ranges between 0.3% and 9.3% in autopsy series.[2] Malignancies and connective tissue diseases with APLA syndrome represent the most common substrates for NBTE. In autopsy studies, neoplasms are the most frequently encountered underlying disease,[3] whereas surgical series have shown that NBTE is more frequent in patients with connective tissue and autoimmune diseases.[4] In a study with 342 SLE patients, NBTE was detected by Doppler echocardiogram in 11% of patients, mostly on the mitral valve.[5] NBTE can occur in any type of cancer except brain tumours.[6] However, cancers of the lung, pancreas and stomach, in addition to adenocarcinomas of unknown primary sites, are the most common malignancies associated with NBTE.[7]
NBTE most commonly affects the aortic valve, and to a lesser extent the mitral valve or both; rarely the right-sided valves are affected.[8] The vegetations in NBTE are sterile and consist of degenerating platelets and fibrin strands without evidence of inflammatory reaction. These vegetations are superficial, and the underlying valvular tissue is either entirely normal or shows subtle histological evidence of abnormal collagen and elastic fibres. The absence of an inflammatory reaction at the site of attachment makes them friable, with a tendency to detach more readily than those present in infective endocarditis. This may lead to infarcts in various organs, including the spleen, kidney, heart and brain. The reported incidence of embolic events is high, i.e. 42% (range 14.1%–90.9%), the most frequent ones being cerebral.[2] In practice, stroke is commonly the first clinical presentation of NBTE.[9]
Diagnosis of NBTE is not easy since the index of suspicion is low and imaging tests have limited diagnostic accuracy. Mckay and Wahler proposed a triad for diagnosis of NBTE—the presence of a disease process known to be associated with NBTE, the presence of heart murmur and evidence of multiple systemic emboli.[10] Typical echocardiographic features of NBTE are described above, but variations are known to occur. While the vegetations in NBTE are generally small, a few case reports have shown relatively larger vegetations. One such patient with APLA was found to have a 1–2 cm mass on AML, which was surgically excised and proven to be NBTE on histopathology.[11] In another instance of a pathologically proven NBTE, the patient had endometrial carcinoma and had presented with systemic thrombosis.[12] Echocardiography done as part of the evaluation showed a 1.5 cm mass on the mitral valve and a few smaller vegetations on the aortic cusps. However, in both patients, the mitral valve vegetations were attached on the atrial aspect of the valve leaflet. In contrast, in our patient, not only the mass was much larger but it was also unusual in its location and morphological appearance.
The most common differential diagnosis of NBTE is infective endocarditis. The vegetations in infective endocarditis generally appear as large, irregular masses on the valve cusps that can extend onto the cords. Unlike NBTE, damage to the underlying valve tissue and extension into the surrounding structures is not uncommon in infective endocarditis. Laboratory parameters can also be useful in distinguishing infective endocarditis from NBTE. Menard et al. suggested that three laboratory tests, namely white blood cell count, CRP and APLA level can help distinguish between these two conditions in most cases.[13] White blood cell count is expected to be low in SLE flare and elevated in infection. CRP is usually elevated in infection, although some elevation may also be seen in active SLE. Elevated APLA titre is also more suggestive of SLE rather than infection, though it cannot exclude concomitant infection. In our patient, none of the laboratory parameters were suggestive of infective endocarditis.
In a valvular mass with frond-like appearance, as seen in our case, papillary fibroelastoma is also a strong possibility. These tumours have a characteristic frond-like appearance resembling a sea anemone. On echocardiography, they appear as small, well-delineated, pedunculated masses which are round, oval or irregular in shape.[14] Half of these tumours have stalks and are mobile. These tumours have a predilection for valves, involving valves in >90% of patients.[14] The aortic valve is most commonly involved, but there is preference for the ventricular surface. In case of mitral valve, however, the atrial surface is the usual site for occurrence of papillary fibroelastoma.[15] There are few case reports involving tricuspid and pulmonary valve. These tumours tend to involve the body of the valve leaflets, mostly in the midportion, and well away from the free edge or the annulus.[15] The diagnosis is usually suspected on the basis of the typical echocardiographic appearance of these lesions. Cardiac MRI can further help in the diagnosis. Thrombus or mass in NBTE will be seen as low-intensity signal, whereas papillary fibroelastoma will be seen as moderate-to-high intensity signal. In our patient, echocardiographic features indicated a papillary fibroelastoma and cardiac MRI seemed to suggest the same diagnosis, even though it was non-confirmatory.
Suspected NBTE is treated for the underlying disease and with anticoagulation. The indications for surgical intervention are not clear but perhaps include recurrent thromboembolism despite therapeutic anticoagulation, acute congestive cardiac failure due to valvular dysfunction and/or persistent uncertainty about the aetiological diagnosis.[16] In our patient, surgery was performed because the patient had presented with a clinically important valve dysfunction that had occurred despite the patient being on a therapeutic dose of warfarin. Given the location and the frond-like appearance of the mass, there was a definite risk of embolism from the mass with potentially devastating complications. Surgery was contemplated because of a persistent diagnostic dilemma not resolved by the noninvasive evaluation, which included echocardiography and cardiac MRI.
Conclusion
This case shows an unusual morphological appearance of vegetation in NBTE and emphasizes that in a patient of APLA syndrome presenting with a mass lesion on the valve, NBTE should remain a possibility, regardless of the morphological appearance of the mass lesion.
Conflicts of interest. None declared
| 1. | el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: Pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–23. [Google Scholar] |
| 2. | Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: A review. Am Heart J 1987;113:773–84. [Google Scholar] |
| 3. | Macdonald RA, Robbins SL. The significance of nonbacterial thrombotic endocarditis: An autopsy and clinical study of 78 cases. Ann Intern Med 1957;46:255–73. [Google Scholar] |
| 4. | Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc 2001;76:1204–12. [Google Scholar] |
| 5. | Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM, et al. Libman-sacks endocarditis in systemic lupus erythematosus: Prevalence, associations, and evolution. Am J Med 2007;120:636–42. [Google Scholar] |
| 6. | Dupeux S, Bricaire L, Bosquet A, Pouchot J, Capron L. Nonbacterial thrombotic endocarditis and gastric carcinoma. Rev Med Interne 2008;29:673–5. [Google Scholar] |
| 7. | Deppisch LM, Fayemi AO. Non-bacterial thrombotic endocarditis: Clinicopathologic correlations. Am Heart J 1976;92:723–9. [Google Scholar] |
| 8. | Biller J, Challa VR, Toole JF, Howard VJ. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol 1982;39:95–8. [Google Scholar] |
| 9. | Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: A diffusion-weighted magnetic resonance imaging study. Stroke 2002;33:1267–73. [Google Scholar] |
| 10. | Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Noninfective valvular masses: Review of the literature with emphasis on imaging techniques and management. Am Heart J 1996;131:1175–83. [Google Scholar] |
| 11. | Vinales KL, Gopalan RS, Lanza LA, Lester SJ, Chaliki HP. Unusual case of nonbacterial thrombotic endocarditis attributable to primary antiphospholipid syndrome. Circulation 2010;122:e459–60. [Google Scholar] |
| 12. | Kaneyuki D, Matsuura K, Ueda H, Kohno H, Kanbe M, Matsumiya G, et al. Surgical management of nonbacterial thrombotic endocarditis in malignancy. Surg Case Rep 2017;3:60. [Google Scholar] |
| 13. | Ménard GE. Establishing the diagnosis of libman-sacks endocarditis in systemic lupus erythematosus. J Gen Intern Med 2008;23:883–6. [Google Scholar] |
| 14. | McAllister HA, Fengolio JJ. Papillary fibroelastoma. In: Landing BH (ed). Tumors of the cardiovascular system. Washington DC:Armed Forces Institute of Pathology; 1978:20–5. [Google Scholar] |
| 15. | Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R, et al. Primary cardiac valve tumors. Ann Thorac Surg 1991;52:1127–31. [Google Scholar] |
| 16. | Rabinstein AA, Giovanelli C, Romano JG, Koch S, Forteza AM, Ricci M, et al. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol 2005;252:352–5. [Google Scholar] |
Fulltext Views
1,568
PDF downloads
223




