Translate this page into:
Adjuvant radiation therapy for malignant tumours of the eyelid: Experience from a tertiary cancer centre in India
2 Department of Surgical Oncology, Dr B.R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Sushmita Pathy
Department of Radiation Oncology, Dr B.R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
drspathy@gmail.com
| How to cite this article: Pathy S, Venkatesulu BP, Mallick S, Deo SS, Mohanti BK. Adjuvant radiation therapy for malignant tumours of the eyelid: Experience from a tertiary cancer centre in India. Natl Med J India 2020;33:271-275 |
Abstract
Background. Tumours of the eyelid are a rare subgroup of neoplasms with varied histology and inherent differences in clinical behaviour. Surgery is the standard of care, and adjuvant radiation therapy (RT) is given in the presence of features suggesting a high risk of local recurrence. The treatment of lymph nodes in the neck is debatable. We reviewed the utility of RT for lymph nodes in the neck in patients with malignant tumours of the eyelid.Methods. We reviewed medical records of all patients with tumours of the eyelid treated at our centre from July 2006 to December 2014 for their demographic, clinical profile, treatment details and outcome.
Results. The records of 37 patients were included for analysis, of these 34 underwent surgery and 21 received adjuvant RT. Their median age was 60 (range 30–85) years. Sebaceous cell carcinoma was the most common (50.4%). The median disease-free survival (DFS) was 35 months (95% CI 17.9–52.0). The 1- and 3-year DFS were 82.7% and 45%, respectively. Univariate analysis showed a superior outcome with early stage (T1) tumours (p=0.01), RT dose of ≥60 Gy and those underwent lymph node dissection (p=0.03). The presence of high-risk factors including close or positive margin had an inferior outcome with a trend towards statistical significance (p=0.06).
Conclusion. We found a favourable outcome with early T stage, RT dose of ≥60 Gy and lymph node dissection. High-risk histopathological features including close margins and positive lymph nodes merit adjuvant RT including regional lymph nodes.
Introduction
Primary carcinoma of the eyelid is an uncommon malignancy and accounts for 5%–10% of all head-and-neck skin cancers. This includes a rare subgroup of neoplasms with varied histology and inherent differences in clinical behaviour. The incidence of tumours of the eyelid differs worldwide with a higher incidence in the USA (15.7 cases/100 000) compared to Asia (5.1/100 000). Basal cell carcinoma is the most common and is reported to be the predominant tumour (80%–90%) among Caucasians. Squamous cell carcinoma represents 3.4%–12.6% and sebaceous cell carcinoma represents 0.6%–10.2% of all head-and-neck skin cancers.[1] In the Indian population, sebaceous histology is common accounting for 2.4%–30.2% of malignant tumours of the eyelid.[2],[3] Compared to basal cell carcinoma, which is slow-growing and insidious, squamous and sebaceous cell carcinoma are more aggressive with a risk of metastasis.[4] Squamous cell carcinomas may masquerade benign clinical conditions such as blepharitis, are often misdiagnosed clinically and present with advanced disease. In addition to being locally aggressive, they are also associated with cranial neuropathy and orbital invasion and a high rate of involvement of regional lymph nodes (24%). Local recurrence rates after surgical resection have been reported to be 2.4%–36.9% at 5 years.[4] Sebaceous cell carcinomas are aggressive with increased metastases to regional lymph nodes (8%–18%) and distant sites (3%–8%). Lymph node involvement has a dismal prognosis.[5],[6],[7]
Surgical excision is the gold standard for curative treatment of primary tumours of the eyelid; however, it can be technically difficult and may result in functional and cosmetic impairment. Radiation therapy (RT) is used either as definitive therapy or in the adjuvant setting in the presence of high-risk features including close/positive margins, invasion of lymphovascular space and involvement of lymph nodes.
Studies on treatment outcomes and optimal treatment strategy are limited due to rarity of the disease. Sebaceous histology is a locoregionally aggressive histology and merits further adjuvant therapy.[8] We analysed our data of tumours of the eyelid and identified the association of various factors in patients treated with surgery with/without RT for tumour response and pattern of failure.
Methods
We reviewed the records of all patients (n=57) with tumours of the eyelid attending the Department of Radiation Oncology from July 2006 to December 2014. We extracted information related to demographic and clinical details, pathological characteristics, RT and outcome of patients with tumours of the eyelid. We excluded 11 patients whose details were incomplete and 9 patients who did not complete their treatment. A total of 37 patients with complete treatment and demographic details were included in the study.
Patients were evaluated and were treated as per the institutional protocol with multimodal therapy. The evaluation protocol involves complete physical examination with visual acuity, laboratory investigations with complete blood count, liver function tests, kidney function tests and computed tomography (CT) of the orbit. The work-up for distant metastasis included chest X-ray and ultrasound of the abdomen. This study was approved by the institute’s ethics committee.
Clinical outcome end-points and statistics
The following demographic data were extracted: sex, age, date of diagnosis, baseline radiological imaging, site of disease, T-and N-stage depending on the histopathology report of the surgical specimen, the number of lymph nodes dissected and positive for tumour deposit.
Disease-free survival (DFS) was calculated from the date of histopathological diagnosis of malignancy till the date of relapse. Relapse was defined as either locoregional recurrence or appearance of new distant metastatic lesion. Patients not having any event at the time of the last follow-up were censored. DFS was estimated using the Kaplan–Meier method. Cox regression was used for univariate analysis for predicting the impact of prognostic factors in DFS. Prognostic variable analysis included histology (sebaceous v. non-sebaceous), size of lesion (<2 v. >2 cm), pathological lymph nodal status, RT dose (<60 v. > 60 Gy) and presence of high-risk features such as close/positive margin. p<0.05 was considered statistically significant. The data were analysed using STATA software version 11.2.
Surgery
Surgery was considered in patients with acceptable oncological and cosmetic outcome and good Eastern Cooperative Oncology Group performance status 0–2. Surgical intervention was based on the size of lesion, visual function and local extent of the disease. Extent of surgery varied from wide local excision in early-stage tumours to orbital exenteration in locally advanced tumours. Soft-tissue reconstruction was done using temporalis muscle flap and local rotation advancement flaps to achieve skin closure. In patients with clinically positive lymph nodes or sebaceous cell carcinoma, a modified neck dissection was done including periparotid lymph nodes and anterolateral group of lymph nodes (level 1 to level 4 lymph nodes). The extent of lymph node dissection was based on intraoperative findings and the clinical judgement of the operating surgeon.
Radiation therapy
Patients with a tumour >2 cm (T3), lymph node-positive disease, close or positive margins, recurrent tumour and/or lympho-vascular space invasion were offered adjuvant RT. Radiation planning was done using two-dimensional simulations with bony landmarks, if treatment was done for the primary alone. Patients were treated in the supine position. A lead piece, 5.5 mm thick with a square window for diseases of the eyelid was made. Eye shield made of tungsten material was placed to protect the cornea after instillation of local anaesthesia. Adjuvant RT consisted of 60–64 Gy in conventional fractionation delivered to the primary site with 9 MeV electrons in CL-2300 linear accelerator (Varian Medical System, Palo Alto, California, United States). If the neck lymph node was positive, it was included in the primary postoperative tumour bed. For conformal planning, gross and clinical target volumes were generated as per the clinical and radiological extent of the disease. Planning target volume was generated with 5 mm margin for daily set-up variation. Based on the reports of lymph nodal metastasis, we adopted a uniform policy in RT planning.
Level 1b, II and intraparotid groups of lymph nodes were contoured according to the RTOG contouring guidelines. Conformal radiation planning was done in Eclipse Planning System (version 6.5) and treatment was delivered with 6-MV photon beam in CL-2300.
Evaluation of treatment response
Patients were monitored during RT with clinical examination and haematological investigations for acute radiation reactions.
Acute adverse events of the skin, conjunctiva and cornea were recorded as per the RTOG guidelines. At our centre, we did a clinical examination every 3 months with CT imaging of the face and neck every 6 months for the first 2 years. Subsequently, clinical examination was done every 6 months with imaging annually or in the presence of clinical suspicion of local recurrence or lymph nodal disease.
Results
Our patients had a median age of 60 years (range 30–85 years). The duration of symptoms ranged from 3 to 60 months with a median of 12 months. There were 20 males and the same number had disease in the right eye [Table - 1].
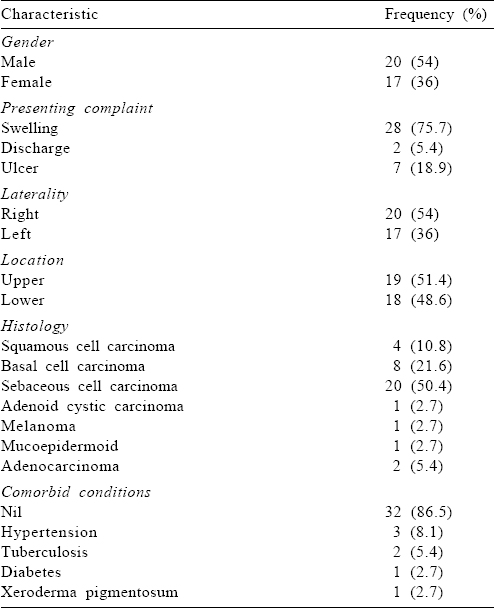
Treatment details
Surgical resection was done in 34 patients, of which 18 underwent wide local excision. Exenteration was performed in 16 patients due to locally advanced disease and 13 patients also had a neck dissection. Twelve patients with sebaceous histology had an upfront neck dissection and 9 had a positive lymph node on histopathology. The remaining 8 patients with sebaceous cell carcinoma underwent wide excision of the primary lesion only. Adjuvant RT was given to 21 patients. Two patients received definitive RT, and 2 patients with advanced disease were treated with palliative RT. Eleven patients received salvage RT after local recurrence.
External beam RT was administered most commonly as adjuvant therapy after surgery. The median dose used for adjuvant RT was 60 Gy (range: 60–64 Gy) [Table - 2].
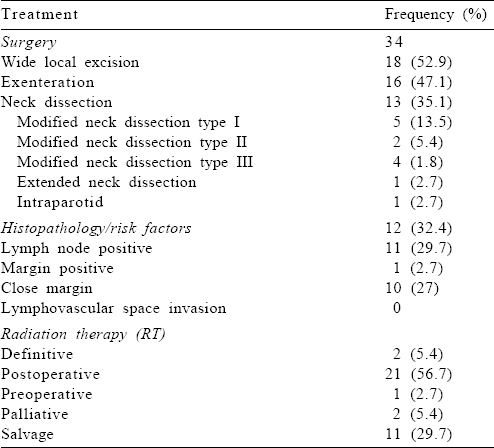
The median number of lymph nodes dissected was 10 (range 1–18). Median number of positive nodes was 1 with a range of 1–5.
Survival outcome
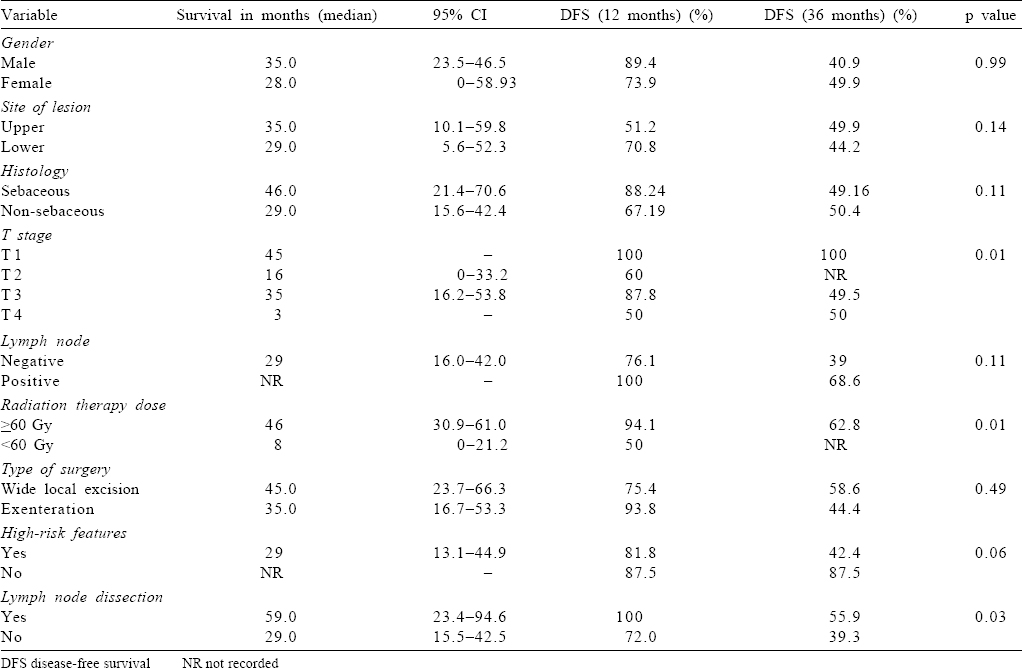
At a median follow-up of 25.3 months (range 1.3–108.7 months), median DFS was 35 months. One- and 3-year DFS were found to be 82.7% and 45%, respectively [Figure - 1]a. Univariate analysis showed a superior outcome with T1 tumour, RT dose ≥60 Gy and those undergoing lymph node dissection (p=0.01, 0.01 and 0.03, respectively; [Table - 3] and [Figure - 1]b,[Figure - 1]c,[Figure - 1]d).
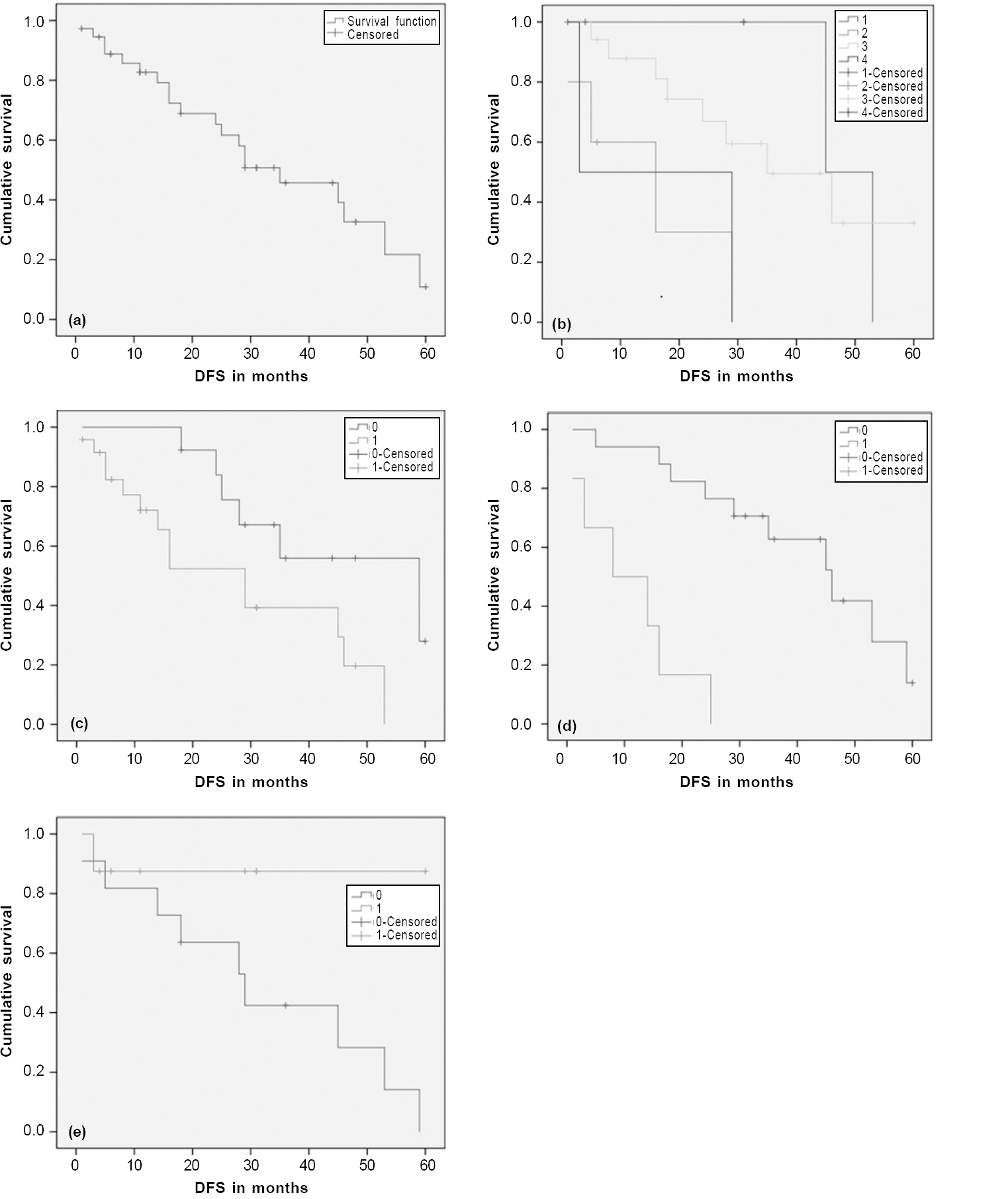 |
| Figure 1: Kaplan–Meier survival curve showing disease-free survival (DFS) of the entire cohort (a); according to the T size (b); according to lymph node dissection (c); according to radiation therapy dose used (d); according to the presence of high-risk features (e) |
The presence of high-risk factors including close or positive margin had an inferior outcome with a trend towards statistical significance (3-year DFS 42.4% v. 87.5%; p=0.06; [Figure - 1]e).
Pattern of recurrence
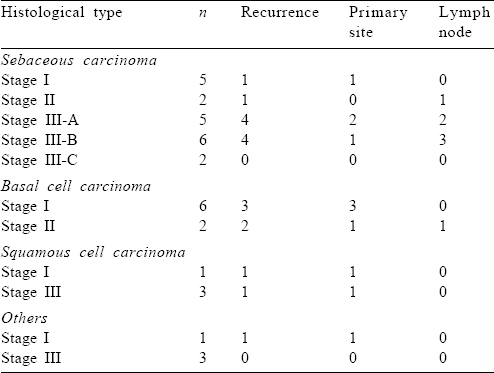
Eighteen patients had recurrence after initial therapy: 11 patients developed local recurrence and 7 had lymph nodal failure. A high rate of lymph nodal failure was observed in the sebaceous cell subgroup: 10 patients developed recurrence, 4 had local recurrence and 6 patients had lymph nodal failure. Four patients with basal cell carcinoma, 2 with squamous and 1 patient with adenocarcinoma had local recurrence [Table - 4].
Salvage treatment
Surgical salvage alone was offered to 7 patients. Surgery and adjuvant RT were offered to 4 patients. Three patients were treated with RT alone (60 Gy) in 30 fractions over 6 weeks. Two patients had disease progression and were administered palliative RT (20 Gy) in 5 fractions in 1 week. Two patients received RT after second recurrence following salvage surgery.
Discussion
Our study evaluated the role of adjuvant RT and the pattern of failure in patients with cancers of the eyelid, who received adjuvant RT. In agreement with earlier reports, we observed an increased incidence of sebaceous cell carcinoma (54%).[6],[9] In our study, the mean age was 60 years, similar to that reported in the literature.[7],[10]
The site of tumour (upper v. lower) and histology (sebaceous v. non-sebaceous) are predictors of survival. However, we did not observe such an association. The high recurrence rate observed by us (18/37) could be because sebaceous histology with locally advanced disease was present in a majority of our patients. The increased failure rate in tumours of the upper eyelid could be due to the high incidence of sebaceous cell carcinoma in this subgroup. The lymph nodal recurrence (6/7) was also high in the sebaceous group. This highlights the aggressive course of sebaceous histology and the need for elective lymph nodal irradiation in the adjuvant setting. The T-category correlates with the risk of lymph nodal and distant metastases; with T2b and larger tumours associated with increased lymph nodal metastasis. Our study observed improved DFS in T1 (median DFS 45 months). However, the survival was superior in T3 than in T2 (median DFS 35 and 16 months, respectively).
Yoon et al. have emphasized the need for negative margins for adequate local control and prevention of recurrence. Our study also highlighted poor outcomes in patients with high-risk features (close/positive margin), with a trend towards statistical significance (p=0.06).[11] The management of regional lymph node metastasis consists of complete dissection of cervical nodes I/II along with parotidectomy.[9],[12]
Thirteen patients underwent lymph node dissection for T3 disease, sebaceous histology and lymph nodal failure/clinically significant lymphadenopathy.
Petsuksiri et al. also showed that primary RT for squamous cell carcinoma of the eyelid provided excellent local control and may be considered as an alternative to surgery.[13] A superior outcome was observed in our study with higher radiation dose in patients receiving ≥60 Gy. Inaba et al. suggested there was a tendency towards favourable outcomes in patients treated with ≥56 Gy and therefore preferred this dose for tumour control.[14] However, whether dose escalation is beneficial for advanced stages needs further larger studies.
Adjuvant RT has a crucial role in optimum locoregional control in the locally aggressive histology (sebaceous cell carcinoma) and presence of high-risk factors including positive margin and lymph node.[15],[16],[17] Currently, there are no consensus guidelines for elective lymph nodal irradiation, and the existing literature on the role of adjuvant RT is limited. In lymph node-positive patients, complete neck dissection followed by adjuvant RT is used for optimal local control. However, there are no data suggesting survival advantage with the addition of RT. Addressing the neck in RT for the eyelid is a debatable issue.
Basal cell carcinoma has an indolent course and is locally aggressive, whereas lymph nodal failure rates are high with sebaceous and squamous variants. Based on our observations in sebaceous and squamous histology and absence of clear high-risk factors to indicate neck irradiation, we suggest it is imperative to consider ipsilateral elective lymph nodal irradiation in locally advanced squamous and sebaceous cell carcinomas.
The median time to recurrence observed was 27.5 months. We suggest long-term surveillance for tumours of the eyelid with meticulous clinical examination and imaging to detect early locoregional failure.
Several factors including a small patient number, clinical heterogeneity and retrospective data are limitations of our study, which preclude us from making more definite conclusions. Multicentric studies are needed to elucidate the efficacy of adjuvant RT for regional metastasis.
Conclusion
Diagnosis and treatment of tumours of the eyelid pose a challenge due to their wide range of clinical presentations, often masquerading benign conditions. Each histological variant is unique in biological behaviour, thus requiring a tailored approach for management.
There is no consensus on RT and dose of regional lymph nodes in tumours of the eyelid, and management strategies are not standardized for regional failure. An RT dose of >60 Gy may be helpful, especially for patients with positive margins. Sebaceous and squamous cell carcinomas have an aggressive clinical behaviour and have an increased propensity to locoregional metastasis. Neck dissection and elective neck irradiation may be considered in this subgroup (>T3 disease).
Conflicts of interest. None declared
| 1. | Cook BE Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: An evidence-based update. Ophthalmology 2001;108: 2088–98. [Google Scholar] |
| 2. | Laskar SG, Basu T, Chaudhary S, Chaukar D, Nadkarni M, Gn M. Postoperative interstitial brachytherapy in eyelid cancer: Long term results and assessment of Cosmesis After Interstitial Brachytherapy Scale. J Contemp Brachytherapy 2015; 6:350–5. [Google Scholar] |
| 3. | Sihota R, Tandon K, Betharia SM, Arora R. Malignant eyelid tumors in an Indian population. Arch Ophthalmol 1996;114:108–9. [Google Scholar] |
| 4. | Kale SM, Patil SB, Khare N, Math M, Jain A, Jaiswal S. Clinicopathological analysis of eyelid malignancies–A review of 85 cases. Indian J Plast Surg 2012; 45:22–8. [Google Scholar] |
| 5. | Silverman N, Shinder R. What’s new in eyelid tumors. Asia Pac J Ophthalmol (Phila) 2017;6:143–52. [Google Scholar] |
| 6. | Deo SV, Shukla NK, Singh M, Jha D, Khanna P, Kallianpur A, et al. Locally advanced sebaceous cell carcinoma (T3) of eyelid: Incidence and pattern of nodal metastases and combined modality management approach. Orbit 2012;31:150–4. [Google Scholar] |
| 7. | Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors. A retrospective study of 5504 cases and review of literature. Am J Dermatopathol 2009;31:256–62. [Google Scholar] |
| 8. | Pardo FS, Wang CC, Albert D, Stracher MA. Sebaceous carcinoma of the ocular adnexa: Radiotherapeutic management. Int J Radiat Oncol Biol Phys 1989;17:643–7. [Google Scholar] |
| 9. | Hashimoto K, Yasumatsu R, Toh S, Shiratsuchi H, Yoshida T, Nishiyama K, et al. Patterns of lymphatic spread and the management of eyelid carcinomas. Auris Nasus Larynx 2016;43:666–71. [Google Scholar] |
| 10. | Chao AN, Shields CL, Krema H, Shields JA. Outcome of patients with periocular sebaceous gland carcinoma with and without conjunctival intraepithelial invasion. Ophthalmology 2001;108:1877–83. [Google Scholar] |
| 11. | Yoon JS, Kim SH, Lee CS, Lew H, Lee SY. Clinicopathological analysis of periocular sebaceous gland carcinoma. Ophthalmologica 2007;221:331–9. [Google Scholar] |
| 12. | Husain A, Blumenschein G, Esmaeli B. Treatment and outcomes for metastatic sebaceous cell carcinoma of the eyelid. Int J Dermatol 2008;47:276–9. [Google Scholar] |
| 13. | Petsuksiri J, Frank SJ, Garden AS, Ang KK, Morrison WH, Chao KS, et al. Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer 2008;112:111–18. [Google Scholar] |
| 14. | Inaba K, Ito Y, Suzuki S, Sekii S, Takahashi K, Kuroda Y, et al. Results of radical radiotherapy for squamous cell carcinoma of the eyelid. J Radiat Res 2013;54: 1131–7. [Google Scholar] |
| 15. | Hata M, Koike I, Maegawa J, Kaneko A, Odagiri K, Kasuya T, et al. Radiation therapy for primary carcinoma of the eyelid: Tumor control and visual function. Strahlenther Onkol 2012;188:1102–7. [Google Scholar] |
| 16. | Connor M, Droll L, Ivan D, Cutlan J, Weber RS, Frank SJ, et al. Management of perineural invasion in sebaceous carcinoma of the eyelid. Ophthalmic Plast Reconstr Surg 2011;27:356–9. [Google Scholar] |
| 17. | Jackson JE, Dickie GJ, Wiltshire KL, Keller J, Tripcony L, Poulsen MG, et al. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: Toward a risk-adapted treatment approach. Head Neck 2009;31:604–10. [Google Scholar] |
Fulltext Views
1,689
PDF downloads
387




