Translate this page into:
Chronic myeloid leukaemia after chemoradiotherapy for solid malignancies
2 Department of Internal Medicine, Trakya University Faculty of Medicine, Balkan Campus 22030 Edirne, Turkey
3 Department of Medical Oncology, Trakya University Faculty of Medicine, Balkan Campus 22030 Edirne, Turkey
4 Department of Medical Genetics, Trakya University Faculty of Medicine, Balkan Campus 22030 Edirne, Turkey
Corresponding Author:
Mehmet Baysal
Department of Haematology, Trakya University Faculty of Medicine, Balkan Campus 22030 Edirne
Turkey
drmehmetbaysal@gmail.com
| How to cite this article: Baysal M, Ulutas G, Gokyer A, Umit E, Atli EI, Kirkizlar O, Gürkan H, Demir AM. Chronic myeloid leukaemia after chemoradiotherapy for solid malignancies. Natl Med J India 2020;33:347-348 |
Abstract
Haematological malignancies associated with chemoradiotherapy (CRT) are often acute myeloid leukaemias and myelodysplastic syndromes. Chronic myeloid leukaemia (CML) has been reported rarely in these situations. Cytogenetics of CRT-associated CML is not different from de novo CML, and there are not enough data about its prognosis. We report two patients who had CRT because of lung cancer and squamous cell carcinoma of head and neck, who subsequently developed CML.Introduction
Chronic myeloid leukaemia (CML) is a stem cell disease caused by uncontrolled proliferation of the myeloid series. Its incidence is 0.7–1/100 000.[1] An abnormal chromosome lies beneath the pathobiology of the disease. A reciprocal translocation occurs between the 9th chromosome and the 22nd chromosome which causes BCR-ABL1 fusion protein and activates tyrosine kinase. This activation leads to clinical manifestations of the disease.[2] Although the exact aetiology of the disease is not known, exposure to ionizing radiations is known to increase the risk of the disease.[3] We present two patients who had received chemoradiotherapy (CRT) for lung and laryngeal cancer and subsequently developed CML.
The Cases
Case 1
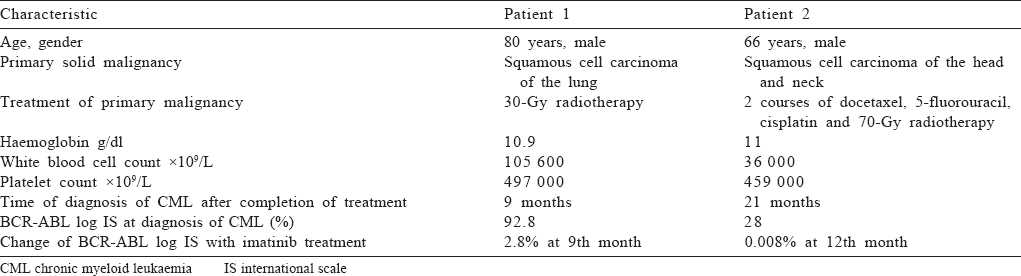
An 80-year-old male presented with cough and dyspnoea. He was found to have a 3 cm mass in his right lung. Bronchoscopic biopsy revealed squamous cell carcinoma of the lung. Because of his age and the geriatric assessment tool results, chemotherapy was omitted. His complete blood counts at first presentation were: haemoglobin 13.2 g/dl, white blood cells 5.3×109/L and platelets 203×109/L. He was given 30-Gy radiotherapy (RT). He presented to us with weakness 9 months after completing his RT. On examination, a closed Traube space was found. His other physical findings were unremarkable. The patient’s laboratory tests were: haemoglobin 10.9 g/dl and white blood cell count was 105 600×109/L (90% neutrophils). In the peripheral blood smear, leucoerythroblastic blood picture was seen, and 14% metamyelocytes, 12% myelocytes, 3% basophil and 2% myeloblast were observed in leucocyte formula. His marrow biopsy revealed a hypercellular marrow with myeloid hyperplasia and no clue of solid malignancy. A cytogenetic abnormality was detected with the karyotype 46, XY, t(9;22)(q34;q11.2) on bone marrow [Figure - 1]. We also observed a BCR-ABL rearrangement in the bone marrow using reverse transcriptase-polymerase chain reaction (RT-PCR); BCR ABL log International Scale (IS) was 92.8%. He was diagnosed with chronic phase CML and Sokal, Eutos and Hasford scores were found to be in the low-risk category. He was started on imatinib mesylate as first-line treatment which he was continuing till the 11th month of his treatment. His 9th month control BCR-ABL log IS result was 2.8%. Fortunately, his lung cancer had stable disease at that time.
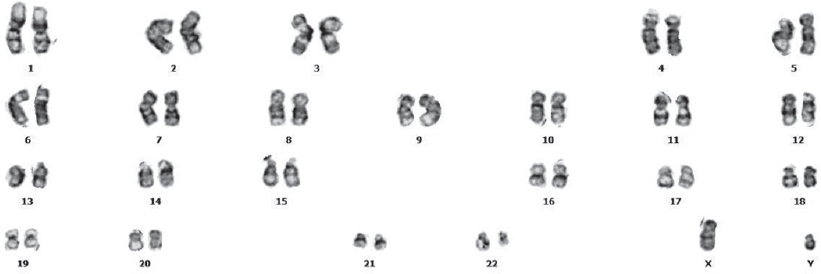 |
| Figure 1: Cytogenetic analysis showing chromosomal translocation (t[9;22][q34;q11.2]) in patient 1 |
Case 2
A 66-year-old male was diagnosed with squamous cell carcinoma in the right jugular region in 2015. He received two courses of docetaxel, 5-fluorouracil and cisplatin chemotherapy followed by radiotherapy 70 Gy to his right cervical region. He had complete response to the treatment. Approximately 21 months after his CRT, he presented with fatigue and exhaustion. His laboratory results were: haemoglobin 11 g/dl, white blood cell count 36.600×109/L and platelet count 459 000×109/L. Myelocytes, metamyelocytes, rods and neutrophils were observed in the peripheral blood smear and basophil 4% and myeloblast 1% were seen. A hypercellular marrow on marrow trephine biopsy showed no signs of infiltration due to metastasis. We did his cytogenetic, fluorescent in situ hybridization (FISH) and molecular examinations on the bone marrow. Philadelphia translocation (t[9;22][q34;q11.2]) was present in all analysed mitoses and no other abnormalities were found [Figure - 2]. PCR revealed a major BCR–ABL gene rearrangement in the peripheral blood and bone marrow cells. RT-PCR BCR ABL log IS was 28%. The patient was diagnosed with chronic phase CML. Sokal, Eutos and Hasford scores were found to be in the low-risk category and imatinib mesylate was started as the first-line treatment. The patient was followed in our haematology outpatient clinic. The BCR ABL log IS value was 0.008% in the first year of his treatment, and a major molecular response was obtained [Table - 1].
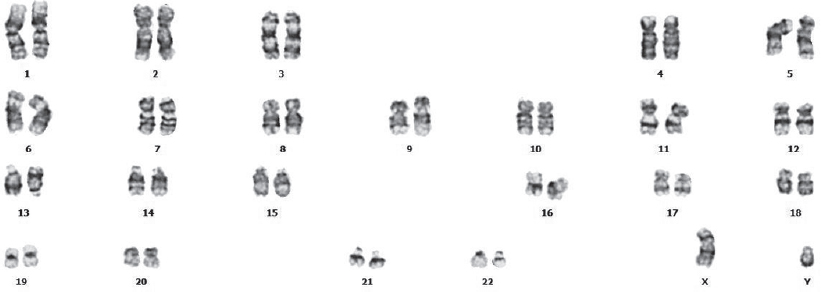 |
| Figure 2: Cytogenetic analysis showing chromosomal translocation (t[9;22][q34;q11.2]) in patient 2 |
Discussion
With more patients being treated with CRT, the number of secondary or treatment-related cancers may increase. The median age at diagnosis of CML is 57–60 years.[1] Ionizing radiation has several possible mechanisms for causing CML; these cause chromosomal aberrations, reactive oxygen species, genomic instability and double-strand DNA breaks.[4],[5] Although ionizing radiation is known to be involved in the aetiology of CML, these findings are generally detected in survivors of atomic bomb attacks and patients who were treated with RT for ankylosing spondylitis.[4],[6] It has been reported that CML was observed in 18% of patients who developed leukaemia after RT for ankylosing spondylitis.[6]
Patients who received RT due to cancer cervix and endometrial carcinoma also have increase in number of cases of leukaemia. This risk is most at 4-Gy dose, but at higher doses the risk decreases.[7] In another study, BCR-ABL1-positive CML cases were found to be increased in patients who received I-131 due to thyroid cancer and those who received RT due to prostate cancer.[8] These case series and reports suggest that CRT may be involved in the aetiology of CML. Therefore, patients harbouring a BCR-ABL1 fusion gene after CRT can be defined as treatment-related CML.[4] There are not enough data about the difference in prognosis between treatment-related or de novo-CML. In addition, there is a pre-clinical silent phase of CML in which the blood counts are normal. The estimated length of the pre-clinical phase is not known; therapy-related cases suggest that CRT may shorten the pre-clinical phase of CML.[4]
The development of acute myeloid leukaemia (AML) after chemotherapy is well-known. Alkylating agents and topoisomerase II inhibitors are two agents that account for the majority of cases with treatment-related AML.[9] Also, cisplatin cross-links in DNA double-strand block DNA repair mechanisms and leads to DNA damage. Therefore, cisplatin also has potential mechanisms to initiate the development of CML.[10] Although CML has been reported after solid tumour-induced chemotherapy,[11],[12],[13],[14],[15] these patients are at greater risk of developing myelodysplastic syndrome (MDS) or AML than CML. These findings can be explained by the presence of many cytogenetic and molecular disorders in the aetiology of MDS and AML, despite the development of CML due to a single genetic disorder. CML is a more homogeneous disease than AML and MDS. CML arises from primitive stem cells and occurs in the early stages of myelopoiesis. It can be argued that CML stem cells are more resistant to DNA damage than AML. In other words, CML stem cells are more frequently resting and therefore less affected by DNA damage.[3],[4]
Conclusion
In patient 1, only RT was used, whereas patient 2 was treated with CRT. CML developed in both the patients. The treatment of patients with CML after CRT does not differ from the treatment of patients with de novo CML. Patients undergoing CRT due to solid tumours are at risk of developing haematological cancers, and rarely CML.
Conflicts of interest. None declared
| 1. | Höglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: An update. Ann Hematol 2015;94 (2 Suppl): S241–7. [Google Scholar] |
| 2. | Goldman JM. Chronic myeloid leukemia: A historical perspective. Semin Hematol 2010;47:302–11. [Google Scholar] |
| 3. | Aguiar RC. Therapy-related chronic myeloid leukemia: An epidemiological, clinical and pathogenetic appraisal. Leuk Lymphoma 1998;29:17–26. [Google Scholar] |
| 4. | Waller CF, Fetscher S, Lange W. Treatment-related chronic myelogenous leukemia. Ann Hematol 1999;78:341–54. [Google Scholar] |
| 5. | Zeidan AM, Long JB, Wang R, Hu X, Yu JB, Huntington SF, et al. Risk of myeloid neoplasms after radiotherapy among older women with localized breast cancer: A population-based study. PLoS One 2017;12:e0184747. [Google Scholar] |
| 6. | Court-Brown WM, Doll R. Leukaemia and aplastic anaemia in patients irradiated for ankylosing spondylitis 1957. J Radiol Prot 2007;27:B15–54. [Google Scholar] |
| 7. | Boice JD Jr., Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst 1987;79:1295–311. [Google Scholar] |
| 8. | Teepen JC, Curtis RE, Dores GM, Berrington de Gonzalez A, van den Heuvel-Eibrink MM, Kremer LCM, et al. Risk of subsequent myeloid neoplasms after radiotherapy treatment for a solid cancer among adults in the United States, 2000-2014. Leukemia 2018;32:2580–9. [Google Scholar] |
| 9. | Fianchi L, Criscuolo M, Fabiani E, Falconi G, Maraglino AM, Voso MT, et al. Therapy-related myeloid neoplasms: Clinical perspectives. Onco Targets Ther 2018;11:5909–15. [Google Scholar] |
| 10. | Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 2014;740:364–78. [Google Scholar] |
| 11. | Dhir N, Zaky W, Zomorodian T, Dhall G. Chronic myeloid leukemia as a second malignant neoplasm in a patient with medulloblastoma after treatment with chemotherapy and irradiation: A case report and review of the literature. Pediatr Hematol Oncol 2013;30:28–9. [Google Scholar] |
| 12. | Lee HY, Lee KH, Hyun MS, Kim MK, Koh SA, Cho HS. Chronic myeloid leukemia as a secondary malignancy after diffuse large B-cell lymphoma. Korean J Intern Med 2014;29:250–2. [Google Scholar] |
| 13. | Demircioglu S. Chronic myeloid leukemia after chemoradiotherapy in a patient with non-small cell lung cancer. Int J Hematol Oncol 2017;27:256–7. [Google Scholar] |
| 14. | Adra N, Sayar H, Einhorn LH. Chemotherapy-related chronic myelogenous leukemia: A case series of patients with germ cell tumor. JAMA Oncol 2016;2:391–2. [Google Scholar] |
| 15. | Kamitori T, Umeda K, Tasaka K, Ogata H, Mikami T, Kato I, et al. Chronic myeloid leukemia following treatment for bilateral retinoblastoma. Pediatr Blood Cancer 2018;65:e27107. [Google Scholar] |
Fulltext Views
1,076
PDF downloads
293




