Translate this page into:
Mitigation of in-hospital risk of coronavirus disease 2019: Experience from a haematology–oncology and stem cell transplant setting
2 Department of Medical Oncology, Dayanand Medical College and Hospital, Ludhiana 141001, Punjab, India
Corresponding Author:
Davinder Paul
Department of Medical Oncology, Dayanand Medical College and Hospital, Ludhiana 141001, Punjab
India
davinder5858@gmail.com
| How to cite this article: Singh S, Paul D, Jain K, Singh J. Mitigation of in-hospital risk of coronavirus disease 2019: Experience from a haematology–oncology and stem cell transplant setting. Natl Med J India 2021;34:10-14 |
Abstract
Background. Coronavirus disease 2019 (Covid-19) was first described in December 2019 and has evolved into an ongoing global pandemic. Cancer patients on chemotherapy are immunocompromised and are at the highest risk of Covid-19-related complications. We describe our experience with the management of haematology–oncology and stem cell transplant (SCT) patients receiving curative chemotherapy in a hospital with a high influx of Covid-19 patients.
Methods. We did a prospective observational study at a 99-bedded cancer centre of a tertiary care teaching hospital from April 2020 to September 2020. Preventive measures taken were categorized as follows: (i) staff: screening, mandatory use of personal protective equipment (PPE), risk stratification of potential exposure and testing and isolation as needed; (ii) patients: mandatory viral polymerase chain reaction testing, segregation of positive and untested patients and testing of family members; and (iii) environment: mandatory regular cleaning, visitor restriction, telemedicine services and reassignment of priority to clinic visits. Treatment of the underlying conditions was continued with added precautions.
Results. A total of 54 patients were included in the analysis, including 48 with haematological malignancies and 6 for stem cell therapy. Preventive measures were universally applied, and chemotherapy with a curative intent was initiated as per protocol. Three patients were detected to have Covid-19 infection before admission and one after the institution of chemotherapy. Nine patients died after the first cycle of chemotherapy, 2 due to severe Covid-19-related illness and 7 due to complications of chemotherapy or disease progression.
Conclusions. In the wake of the Covid-19 pandemic, treatment for haematological malignancies must continue while balancing the risk of Covid-19 infections. Our report emphasizes the effectiveness of measures such as hand hygiene, social isolation, patient segregation, use of masks and PPE and universal pre-treatment testing for Covid-19 in reducing the risk of infection in a high-risk clinical setting.
Introduction
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is an RNA virus first described in the context of a severe respiratory illness in China in December 2019.[1] Two related strains, the SARS-CoV and Middle East respiratory syndrome CoV, have already been associated with human epidemics.[2],[3] Covid-19 can present from an asymptomatic carrier state to respiratory failure and multi-organ dysfunction.[4] Up to 14% of patients present with severe hypoxic illness and 5% with multi-organ dysfunction require admission to an intensive care unit (ICU).[5] The risk of severe disease and mortality with Covid-19 is increased 3–4 times in patients with cancer, especially those requiring high-dose chemotherapy followed by prolonged neutropenia.[6] Several guidelines have been published on priority which recommend delaying or de-intensifying treatment if possible. However, this may not be possible in a number of patients undergoing treatment for haematological malignancies and stem cell transplantation (SCT) due to fear of disease progression. Treatment in this setting requires attenuation of infective risk of SARS-CoV-2 to patients, caregivers and healthcare professionals while continuing treatment in a high-risk environment. We present our experience in a haematology/oncology and SCT service in the midst of the Covid pandemic.
Methods
This was a prospective observational study done in the cancer unit of a 1600-bed tertiary care teaching hospital in northern India. The adult cancer treatment services are located in a separate building in the campus with 99 dedicated beds, with outpatient services on the ground floor, inpatient wards on the 1st to 3rd floors, leukaemia/transplantation wards on the 4th floor and surgical oncology on the top floor. All adult patients diagnosed to have haematological malignancies from 1 April 2020 to 15 September 2020, requiring inpatient chemotherapy for acute leukaemia, lymphoma, multiple myeloma and SCT were included in the analysis. Patients who were treated on an outpatient basis (e.g. chronic myeloid leukaemia, multiple myeloma or lymphoma) or kept on observation alone (e.g. low-grade lymphoma) were excluded from the analysis.
After declaration of the Covid pandemic, lockdowns were imposed in most parts of the country, leading to an obligatory change in practice patterns. Many patients on oral therapy or chemotherapy for lymphoma or myeloma were seen at longer intervals. The SCT service was put on hold, but chemotherapy for leukaemia and high-grade lymphoma was carried on as usual based on the discretion of the treating physician. In view of clinical urgency of certain patients, the SCT service was re-initiated with mandatory precautions from June 2020 after approval from the hospital administration.
Treatment protocols
Standard treatment protocols were used for the treatment of haematological malignancies, selected at the discretion of the primary physician. The protocols for initiation of therapy are summarized as follows:
- Acute myeloid leukaemia (AML): Induction with ‘7/3’ protocol including daunorubicin 60 mg/m2 for 3 days and cytarabine 100 mg/m2 for 7 days for fit patients <60 years of age. Elderly or unfit patients were initiated on treatment with azacytidine 75 mg/m2 for 7 days, with or without venetoclax.
- Acute lymphoblastic leukaemia (ALL): Induction with a Berlin–Frankfurt–Muenster- or German Multicentre ALL-based protocol depending on patient age was followed. Adults with relapsed or high-risk ALL were planned for treatment intensification with the hyper-cyclophosphamide, vincristine, adriamycin and dexamethasone protocol
- Lymphoma: The preferred first-line treatment for diffuse large B-cell lymphoma and Hodgkin lymphoma was rituximab, cyclophosphamide, adriamycin, vincristine and prednisolone and adriamycin, bleomycin, vinblastine and dacarbazine, respectively. Management of patients with lymphoma was based on the Indian Expert Consensus guidelines.[7]
- Stem cell transplantation: The standard preparative regimen for plasma cell dyscrasias was high-dose melphalan and for lymphoma was BEAM (BCNU, etoposide, cytarabine and melphalan). Allogeneic protocols were selected on an individual basis.
Consistent with the departmental policy, full doses of all chemotherapeutic agents were administered and no dose reduction was done routinely. Febrile neutropaenia was managed according to standard protocols, and a high-resolution computed tomography (HRCT) scan of the chest was done for patients with acute leukaemia with fever lasting beyond 72 hours. For patients with respiratory symptoms who were initially Covid-19 negative, a repeat reverse-transcriptase-polymerase chain reaction (RT-PCR) and HRCT chest were done after 7 days.
Measures taken to reduce Covid-19 risk
These were broadly divided into the following categories:
Healthcare staff
- Personal protective equipment (PPE): All physicians and nurses were provided plastic-fronted gowns, N95 masks, nitrile gloves and plastic face shields. A pack of four new masks was provided every 2 weeks to each physician, and gowns and gloves were renewed daily. Janitorial staff were also provided gloves, masks and plastic gowns.
- Staff screening: A counter was set up at the entrance of the building and all staff were screened by infrared thermometers (Shenzen Bliss, NC-9900 model). A history of travel, contact with a known Covid-19 patient and respiratory symptoms was obtained mandatorily from all staff reporting for duty.
- Potentially infected staff: The Indian Council of Medical Research (ICMR) provides guidelines for categorization of infection risk after a potential exposure with an infected individual.[8] Staff members with potential exposure were classified as low risk or high risk depending on the use of PPE and duration of contact. Low-risk contacts were allowed to continue working with self-monitoring of symptoms, whereas high-risk contacts were quarantined for 14 days and tested as per the ICMR guidelines.
Social distancing
- Telemedicine consults: Social distancing was practised by reducing the inflow of routine clinic patients in the outpatient services. A tele-consultation facility was set up as per the Ministry of Health and Family Welfare guidelines, where patients could remotely connect with the reception staff who managed queries and consultations in liaison with the primary physicians. Patient appointments were reorganized so that only patients with an urgent or time-sensitive need of consultation were called to the outpatient clinic.
- Restriction of numbers: The number of patients visiting the outpatient clinic was restricted, and seating was arranged to ensure a minimum recommended gap between two patients and their attendants.
In-hospital
- Mandatory testing: All patients admitted in the cancer wards were compulsorily tested for Covid-19 by a naso-pharyngeal RT-PCR or rapid antigen method before or at admission in the hospital. This was also practised in the rest of the hospital so that there was no risk of iatrogenic spread of infection.
- Social isolation: Till the reports were available, patients were admitted in a pre-Covid ward before being shifted to the designated wards to make sure there was no mixing of Covid-positive and -negative patients. Only one attendant was allowed to enter the hospital with each patient.
- Patients with respiratory symptoms: For patients who developed respiratory symptoms or hypoxia while in the hospital, a policy of performing a repeat HRCT chest and a Covid-19 RT-PCR was followed. Patients were shifted to the pre-Covid ward till the results of Covid RT-PCR and HRCT chest were available.
- Duration of stay: Patients were kept admitted till recovery of counts (absolute neutrophil count >1000/μl). As patients with acute leukaemia and transplant were admitted for >2 weeks, additional precautions were needed. This included strict implementation of one-visitor policy, daily screening with an SpO2 monitor and admission in a different area from patients with short admissions.
Stem cell transplant unit
- Testing of donor and family: Recipients and donors were routinely tested before the initiation of conditioning chemotherapy. Although there are no recommendations for testing family members, we adopted a policy of testing selected family members so that they could be allowed to visit patients in the SCT unit after detailed discussion with the families.
- Restriction of visitors: With the exception of children, no visitors were allowed inside during the period of inpatient stay. Video calling through cell phone or iPad was allowed.
- Environmental cleaning: Wet mopping of floors with a 10% hypochlorite solution was ensured once every 6 hours. An 8% solution of didecyl-dimethyl-ammonium-chloride (Virex®) was used to clean the door handles and hard surfaces inside the bone marrow transplant unit at least thrice a day.
Post-discharge
- Priority follow-up: Patients discharged after high-dose chemotherapy or SCT were given appointments 1–2 hours before regular clinics started to prevent overcrowding and infection risk.
- Day-care limitation: The day-care chemotherapy schedules were limited by 75% to prevent overcrowding. As the day-care area is shared by medical oncology, individual decisions were made to defer chemotherapy for non-urgent indications to reduce crowding. Any injections or blood transfusions for post-discharge patients were attempted to be started before the beginning of routine outpatient clinics.
Results
Treatment details
A total of 54 patients were included in the analysis, of which 31 (57.4%) were men. The median age of the entire cohort was 43 (range 11–77) years. Patients aged >60 years constituted 26% (n=14) of the cohort. Long-term comorbid conditions were present in a total of 16 (29%) patients, including 6 with diabetes mellitus, 7 with systemic hypertension and 3 with cardiac disease. The final diagnosis consisted of AML in 10 patients (18.5%), ALL in 14 (25.9%), lymphoma (all categories) in 22 (40.7%) and multiple myeloma in 2 patients. Six patients (11.1%) were admitted for SCT [Table - 1].
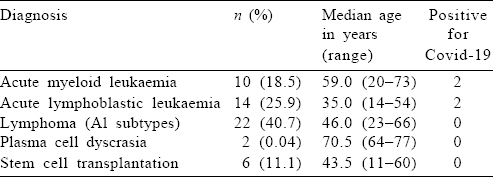
Chemotherapy was started at full dose with a curative intent for 52 (96.2%) patients [Table - 2]. Two patients were detected to have Covid-19 at diagnosis and underwent modification of therapy (described below).
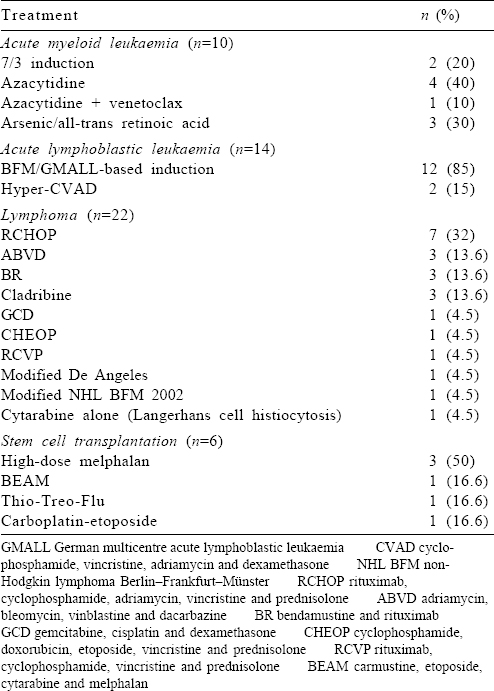
Six of the 10 patients with AML had an HRCT chest as part of febrile neutropenia protocol, and 1 patient had a repeat nasopharyngeal swab for Covid-19 RT-PCR in view of respiratory symptoms, which was positive.
For the entire cohort, the mean (SD) number of packed cell transfusions was 2.2 (5.8) and platelet concentrates was 5.2 (17.4). The maximum number of red cell and platelet concentrates received was 9 and 42, respectively, for patients with ALL. The donors were not screened routinely for Covid-19, and no apparent symptomatic infection after blood product transfusion was observed.
Covid-19-positive patients
Four patients were positive for Covid-19 infection by nasopharyngeal RT-PCR, 3 before initiation of therapy and 1 after starting treatment [Table - 3]. Of the patients with preadmission Covid-19 positivity, 2 were detected incidentally and 1 was acutely sick with pneumonia and type 1 respiratory failure. The first patient (14-year-old boy) was admitted with B-ALL, for whom prednisolone and vincristine were started as per protocol, but the first dose of daunorubicin was omitted. The second patient (20-year-old man) was found to be positive after completing AML induction and before starting the first cycle of HiDAC. Both patients were subsequently started on full-dose chemotherapy uneventfully after a negative RT-PCR 2 weeks later. The third patient (27-year-old woman) presented with acute promyelocytic leukaemia and bilateral pneumonia with type I respiratory failure at admission for which she required intubation and mechanical ventilation. The fourth patient (35-year-old woman) was admitted with a negative RT-PCR and started on abbreviated 7/3 chemotherapy for AML. She developed fever and hypoxia on day 15 of chemotherapy with a total white blood cell count of 100/μl A repeat HRCT showed features consistent with severe Covid infection (CO-RADS score V), and she succumbed to the same after 48 hours. None of the 2 patients who died with Covid-19 had any prior comorbid conditions [Table - 3].
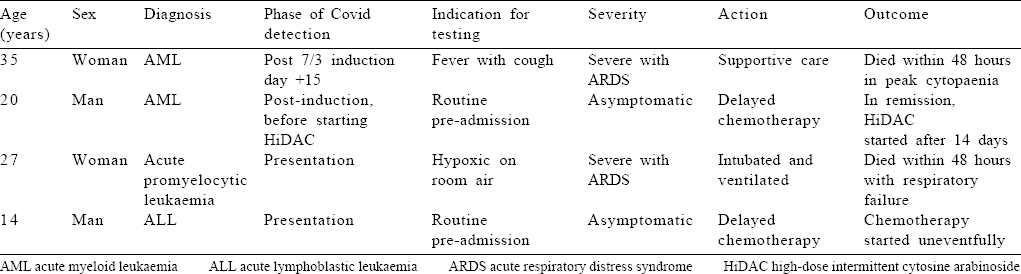
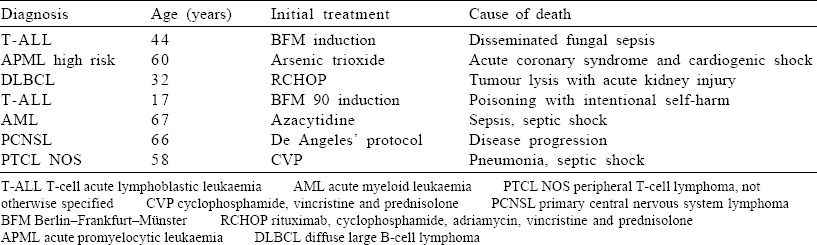
Overall, 9 patients (16.6%) died after the first cycle of chemotherapy [Table - 4], including 2 with severe Covid infection and 7 with unrelated complications. The remaining 45 patients are well and on regular follow-up. All the patients who underwent SCT are also doing well on regular follow-up and monitoring.
Discussion
The Covid-19 pandemic is an ongoing global health crisis and has led to over 974 000 deaths over an 8-month period. This unprecedented predicament has adversely affected a number of aspects of healthcare delivery, including cancer care.[9] While non-urgent medical services and procedures are being deferred in multiple institutions, treatment of emergent medical conditions and several malignancies continues. Haematological malignancies, especially acute leukaemia, fall in the latter category and need to be treated aggressively to improve patient survival and quality of life. The threat of Covid-19 affects this paradigm due to delays in diagnosis and treatment and shortage of blood products.[10] Various collaborative groups have issued guidelines for the prevention and reduction of Covid-19 risk in the cancer care setting. It is essential to adapt these guidelines for local use so that healthcare delivery can continue with utmost safety and efficiency.
An elevated risk of severe illness and mortality with Covid-19 in patients with cancer is evident from several reports. In a dataset from New York, the case fatality ratio for Covid infections in patients with cancer was noted to be two-fold higher than in patients without cancer.[11] A meta-analysis including 46 499 patients from 32 studies reported a higher risk of mortality (relative risk [RR] 1.66, 95% CI 1.33–2.07) and probability of ICU admission (RR 1.56, 95% CI 1.31–1.87) in this group.[12] Patients who receive chemotherapy up to 14 days before the onset of infection have been noted to have a much higher incidence of severe illness and complications (HR 4.079, 95% CI 1.086–15.322).[13] Consistent with these data, we deferred intensive chemotherapy for 2 asymptomatic patients who had a positive RT-PCR at diagnosis.
Certain epidemiological characteristics of Covid-19 can thwart efforts to prevent person-to-person transmission. First, up to 40%–45% of patients are asymptomatic and can transmit the infection to more vulnerable populations.[14] Second, the period of maximum infectivity can peak 2–3 days before the onset of symptoms, making it difficult to isolate potentially infective individuals.[15] Third, patients who need regular access to healthcare, including those with diabetes mellitus, hypertension, cardiac disease, chronic kidney disease and malignancy, are ones at the highest risk of severe disease and mortality.[16] These attributes underscore the implementation of universal measures for infection control and prevention. Consistent with this, documentation of contact and travel history was obligatory for all staff, patients and visitors coming to our centre. Those with a history of foreign travel, fever and upper respiratory symptoms were referred to the infectious disease unit for further assessment and risk stratification.
We followed a practice of screening all patients with an inhouse SARS-CoV-2 RT-PCR before admission. This is in keeping with the Infectious Disease Society of America guidelines which recommends testing of all patients before immuno-suppressive therapy.[17] Patients planned for admission were kept in a pre-Covid area till reports of RT-PCR testing were available. As the average incubation period is 4–5 days, it is possible for an infected patient to test negative and manifest the disease at a later date.[18] To avoid missing these cases, we adopted a policy of re-testing after a 7-day interval for those with fever or respiratory symptoms. One of our patients who tested negative and later developed Covid-19 pneumonia perhaps falls in this category.
Due to a high risk of complications, chemotherapy should be started immediately only if the benefit of starting treatment outweighs the risks. A framework for deciding priority of treatment is provided by Cancer Care Ontario, which recommends classifying patients into three levels of priority. The highest priority is assigned to diseases where treatment delay adversely affects the survival or quality of life and include lymphoma and leukaemia.[19] This is mirrored in our protocols, and treatment was not deferred for any patient except those positive for Covid-19 at diagnosis.
Sparse data are available on the effects of Covid-19 on SCT recipients. The risk of viral respiratory infections is elevated from the pre-engraftment phase to the late post-engraftment phase (more than 100 days post-transplant).[20],[21] This delayed elevation of risk is related to immunosuppressive medications, graft-versus-host disease and delayed immune reconstitution.[22] Guidelines for prevention and management of Covid-19 in the transplant setting were published online on 28 July 2020, by the American Society of Transplantation and Cellular Therapy; in retrospect, most of our practices are broadly in tune with the same.[23]
The transplantation procedure requires a collaborative effort by multiple professionals, including physicians, nurses, janitorial staff, dieticians and pharmacists. With the exception of children, a family member is usually not allowed to stay inside the SCT unit as daily visits carry a potential risk of transmitting infection. None of the recipients tested positive before admission to the SCT unit. In case a recipient tests positive for Covid-19, a deferral of at least 3 months is considered ideal. The European Bone Marrow Transplant guidelines acknowledge the urgency of SCT for certain conditions, and a 2–3-week deferral with 2 negative Covid-19 RT-PCR is acceptable for high-risk diseases.[24] We followed a similar policy and tested patients a maximum of 48 hours before admission for SCT.
Both the European Bone Marrow Transplant and American Society of Transplantation and Cellular Therapy guidelines provide similar advice regarding donor testing. For a donor who tests positive, a 3-month deferral is recommended. A more common scenario is a donor who has a presumed contact with an infectious patient, which warrants a minimum deferral of 28 days. We followed our institutional policy of assessing donor risk and advising social isolation for at least 2 weeks before the procedure. The donor was also tested with a RT-PCR a maximum of 48 hours before starting conditioning chemotherapy for the patient.
Detection of viral RNA in plasma during active infection raises the possibility of blood-borne transmission. However, its significance is uncertain as no correlation has been found between plasma viral loads and disease severity.[25] The risk of blood-borne transmission of Covid-19 has been found to be extremely low, and transfusion practices with universal precautions and donor screening are found to be safe.[26] The American Association of Blood Banking and FDA guidelines are in concurrence and advise against any additional measures for blood collection and testing.[27] None of our patients developed clinical or laboratory-proven Covid-19 infection despite receiving an average of 2.2 packed red cell and 5.2 platelet concentrates.
Covid-19 is an unprecedented global emergency, and our understanding of the disease is evolving as we write. Prevention is paramount to bringing the pandemic under control, and its role is magnified further in a setting where treatment-related immunosuppression is inevitable. Our hospital is currently playing a key role in community education, testing and management of patients with Covid-19. As of July 2020, approximately 12 000 RT-PCRs have been done in our institution, of which 1100 were positive. In an unprecedented step, more than 300 beds in the hospital have been re-purposed and dedicated to care of Covid-19-positive patients. Due to a high influx of potentially infected patients, stringent guidelines were implemented to reduce infection for the most vulnerable patients.
Our report emphasizes the utility of basic measures such as hand hygiene, masks, social distancing and pre-treatment testing for Covid-19 risk reduction in a high-risk clinical setting. This allowed us to achieve strict infection control for patients on active chemotherapy with no excess mortality and will expectantly serve as a blueprint for future infection control.
Conflicts of interest. None declared
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [Google Scholar] |
| 2. | Su S, Wong G, Shi W, Liu J, Lai AC, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24: 490–502. [Google Scholar] |
| 3. | Chafekar A, Fielding BC. MERS-CoV: Understanding the latest human coronavirus threat. Viruses 2018;10:93. [Google Scholar] |
| 4. | Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, et al. Clinical outcomes of COVID-19 in Wuhan, China: A large cohort study. Ann Intensive Care 2020;10:99. [Google Scholar] |
| 5. | Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72/314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [Google Scholar] |
| 6. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 2020;21:335–7. [Google Scholar] |
| 7. | Nair R, Kakroo A, Bapna A, Gogia A, Vora A, Pathak A, et al. Management of lymphomas: Consensus document 2018 by an Indian expert group. Indian J Hematol Blood Transfus 2018;34:398–421. [Google Scholar] |
| 8. | Mahalmani VM, Mahendru D, Semwal A, Kaur S, Kaur H, Sarma P, et al. COVID-19 pandemic: A review based on current evidence. Indian J Pharmacol 2020;52:117–29. [Google Scholar] |
| 9. | Parodi SM, Liu VX. From containment to mitigation of COVID-19 in the US. JAMA 2020;323:1441–2. [Google Scholar] |
| 10. | Gavillet M, Carr Klappert J, Spertini O, Blum S. Acute leukemia in the time of COVID-19. Leuk Res 2020;92:106353. [Google Scholar] |
| 11. | Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 2020;10:935–41. [Google Scholar] |
| 12. | Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: A meta-analysis of patient data. JCO Glob Oncol 2020; 6:799–808. [Google Scholar] |
| 13. | Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. [Google Scholar] |
| 14. | Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med 2020;173:362–7. [Google Scholar] |
| 15. | He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–5. [Google Scholar] |
| 16. | Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance–United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759–65. [Google Scholar] |
| 17. | Hanson KE, Caliendo AM, Arias CA, Englund JA, Lee MJ, Loeb M, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19. Clin Infect Dis 2020 Jun 16:ciaa760. doi: 10.1093/cid/ciaa760. [Google Scholar] |
| 18. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [Google Scholar] |
| 19. | Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: A comprehensive review. Curr Oncol Rep 2020;22:53. [Google Scholar] |
| 20. | Khanna N, Widmer AF, Decker M, Steffen I, Halter J, Heim D, et al. Respiratory syncytial virus infection in patients with hematological diseases: Single-center study and review of the literature. Clin Infect Dis 2008;46:402–12. [Google Scholar] |
| 21. | Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019;68:e1–e47. [Google Scholar] |
| 22. | Alexandersson A, Koskenvuo M, Tiderman A, Lääperi M, Huttunen P, Saarinen-Pihkala U, et al. Viral infections and immune reconstitution interaction after pediatric allogenic hematopoietic stem cell transplantation. Infect Dis (Lond) 2019;51:772–8. [Google Scholar] |
| 23. | Waghmare A, Abidi MZ, Boeckh M, Chemaly RF, Dadwal S, El Boghdadly Z, et al. Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant 2020;26:1983–94. [Google Scholar] |
| 24. | Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant 2020;55:2071–6. [Google Scholar] |
| 25. | Joynt GM, Wu WK. Understanding COVID-19: What does viral RNA load really mean? Lancet Infect Dis 2020;20:635–6. [Google Scholar] |
| 26. | Chang L, Yan Y, Wang L. Coronavirus disease 2019: Coronaviruses and blood safety. Transfus Med Rev 2020;34:75–80. [Google Scholar] |
| 27. | Committee ATTD. Update: Impact of 2019 Novel Coronavirus and Blood Safety AABB; 2020. [Google Scholar] |
Fulltext Views
2,041
PDF downloads
680




