Translate this page into:
Covid-19 Vaccines: Several technologies at work
2 Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India
Corresponding Author:
Rakesh Aggarwal
Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006
India
aggrawal.ra@gmail.com
| How to cite this article: Aggarwal A, Aggarwal R. Covid-19 Vaccines: Several technologies at work. Natl Med J India 2021;34:1-3 |
Immunization is an important tool in our armamentarium for the prevention of infectious diseases, and morbidity and mortality associated with them. Immunization, through inoculation of material taken from persons with smallpox, is believed to have been used in China and India as far back as the 15th century.[1] However, vaccination, in its current form, began in 1796 when Edward Jenner used the cowpox material to induce protection against smallpox. Vaccination has had major successes, most notably the eradication of smallpox during the 1970s, and the near eradication of polio. In recent years, it has helped control the outbreaks of Ebola virus. The WHO estimates that the immunization programmes save nearly 2–3 million lives each year. Vaccination is currently the focus of attention for the medical profession and general public alike, because of the development and deployment of vaccines to control the ongoing Covid-19 pandemic, caused by severe acute respiratory disease coronavirus 2 (SARS-CoV-2).
A vaccine is a biological product that induces an acquired immune response directed specifically against a particular pathogen, and thus confers protection against infection and/or disease on subsequent re-exposure to it. To do so, it must contain either the complete pathogen or one or more of its components [Table - 1]. It may also contain adjuvants, which serve to enhance the immune responses, stabilizers, preservatives and buffers.
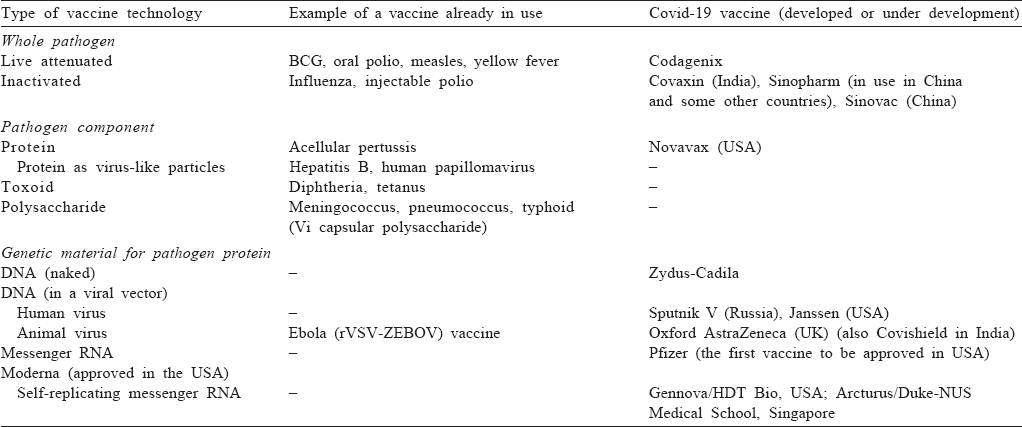
The vaccines that contain the whole pathogen are broadly of two types––live (attenuated) and non-live (killed or inactivated). A live vaccine contains a replicating micro-organism that shares cross-protection against the pathogen but lacks its virulence. It can be either a non-pathogenic organism closely related to the pathogen or a modified strain of the latter. Such ‘attenuated’ vaccines provoke a strong and long-lasting immune response. However, they carry the risk, usually small, of multiplication and disease in immunocompromised persons and of mutation into a virulent form. The killed vaccines contain the pathogen itself, which has been rendered incapable of multiplication through heat, chemical treatment or radiation exposure.
Alternatively, a vaccine can contain an immunogenic component (antigen[s]) of the pathogen. Since antigens are mostly proteins, the vaccines contain either one or more native proteins derived from the pathogen, or a protein (or a part of it) synthesized using recombinant technology. In case of viruses, these often take the form of virus-like particles (VLPs), which consist of viral proteins self-assembled into particles that mimic the native architecture of the virus. Their particulate structure with repetitive, high-density surface display of the viral protein helps enhance the uptake of protein by the immune cells and thus in eliciting stronger immune responses. At times, especially for bacteria, other pathogen components, such as toxoids (toxins which are inactivated using heat or a denaturant such as formaldehyde) and polysaccharides (alone or as protein conjugates), are used as antigens.
As would be expected, each of these traditional approaches has been used in the attempt to develop vaccines against SARS-CoV-2. The front runners for the killed vaccine approach are Covaxin (Bharat Biotech) in India,[2] and Sinopharm and Sinovac in China.[3],[4] The former is ready for launch in India, and the latter two are already in use in China and some other countries. A live-attenuated vaccine is being co-developed by Codagenix (USA) and Serum Institute of India, as a single-dose intranasal vaccine, and a phase 1 trial for it has begun recently.[5] Vaccines containing SARS-CoV-2 spike protein developed by Novavax and the Center for Genetic Engineering and Biotechnology of Cuba are in the clinical trial stage; Biological E, an Indian company, is also developing a protein-based vaccine.[6],[7]
The main change in the context of SARS-CoV-2 vaccines has been the use of newer platforms that involve de novo synthesis of antigen in the body of the recipient. These are based on our knowledge of the central dogma of biology that genetic message encoded in DNA is transferred in a unidirectional way to mRNA by transcription, and further to protein synthesis through translation. These newer technologies use DNA—either naked or carried inside a viral vector—or RNA, which contains the genetic information for the production of a key immunogenic protein of the pathogen, instead of a pathogen or its protein.
DNA-based vaccines mostly use viral vectors as the route of delivery. These vectors are able to deliver DNA coding for a pathogen protein into the host cells, whose machinery then uses it to synthesize the desired antigen. Initially, relatively non-pathogenic live viruses, such as adenoviruses, were tried as vectors. However, currently, replication-deficient viruses are preferred in view of their lack of adverse effects related to multiplication of the carrier virus. Even before the Covid-19 pandemic, this approach had been successfully used to develop two vaccines against Ebola virus. This strategy has now been used successfully to develop a Covid-19 vaccine by the Oxford Group in the UK, in collaboration with AstraZeneca, using a replication-deficient chimpanzee adenovirus backbone;[8] this vaccine is also likely to be available in India soon, as Covishield. Sputnik V vaccine from Gamaleya, a Russian company, also uses this technology.[7] The downside of this technology is that prior exposure and hence immunity to the vector virus in the vaccine recipient may diminish the vaccine’s immunogenicity. Further, for the same reason, the booster doses may elicit a weaker response.
Alternatively, naked DNA coding for the desired antigen can be given by intradermal or intramuscular route using a gene gun—a technology being explored by Zydus-Cadila in India.[7] DNA vaccines have the advantages of ease of production in large quantities, storage at room temperature, and needle-free delivery (using gene gun) leading to better acceptance, especially among children. However, these vaccines may not produce an adequate amount of antigen, if the delivered DNA is extruded from the cell, and may need multiple doses. Also, administration of DNA carries a risk, at least theoretical, of its integration into the host genome.
The real novel development in the past one year has been that of RNA-based vaccines. Messenger RNA (mRNA) is the molecule that carries the genetic message from DNA in the nucleus to the cytoplasm for production of proteins. It can thus be used as the template for production of an antigen of interest, just like DNA. Though the administration of mRNA was shown to lead to in vivo production of proteins in early 1990s,[9] its use was limited by concerns about its instability, inefficient in vivo delivery into the host cells and high innate immunogenicity.[10] However, in the meanwhile, modification techniques have been developed that increase the stability of mRNA and down-modulate its ability to activate Toll-like receptors and hence reduce the adverse events related to type I interferon response. Further, efficient in vivo delivery has been achieved by formulating mRNA into carrier molecules, such as lipid nanoparticles, which permit its rapid uptake into the host cells and expression in the cytoplasm. With the removal of these barriers, mRNA has emerged as a low-cost, highly-scalable, non-infectious and non-integrating vaccine platform. Their main disadvantage is the need to be transported and stored at ultra-low temperatures, limiting their use in resource-constrained settings. Thus, for the first time, two mRNA-based vaccines against SARS-CoV-2 have been developed by Pfizer-BioNTech and Moderna. Both of these have excellent efficacy and strong immunogenicity and efficacy against clinical Covid-19,[11],[12] and have been approved and introduced in the USA and Europe. An additional potential advantage of these vaccines is the ease of modification in case of emergence of variants––a risk for several pathogens, in particular RNA viruses, such as influenza and potentially SARS-CoV-2. The strong immunogenicity of the Covid-19 mRNA vaccines is likely to lead to attempts in using this technology to improve upon the existing vaccines against other diseases as well.
There is more to come. To further increase the degree and duration of antigen expression, strategies are being tried to make RNA replicate inside the host cells. Thus, a self-amplifying RNA that codes for both the antigen of interest and an RNA replicase is being explored by Gennova, an Indian company, in collaboration with USA-based HDT Bio, as also by Arcturus Therapeutics in collaboration with Duke-NUS Medical School, Singapore.[7] Alternatively, a mixture of two mRNAs—one coding for the antigen of interest and another for replicase—can be used.
What is noteworthy is the speed with which these vaccines have been developed— with several vaccines having been approved and used within less than a year of the discovery of SARS-CoV-2. This has been possible because of partnerships between various stakeholders, viz. academic scientists, pharmaceutical companies, government and non-government agencies pledging funds, and above all the community that willingly participated in trials on vaccines with previously unexplored platforms.
These advances make it possible that, in the foreseeable future, we will be able to develop vaccines for other infections that have previously been difficult to tackle, as also against cancer and other chronic diseases.
| 1. | Wikipedia. Variolation. Available at https://en.wikipedia.org/wiki/Variolation (accessed on 7 Jan 2021). [Google Scholar] |
| 2. | Mohandas S, Yadav PD, Shete-Aich A, Abraham P, Vadrevu KM, Sapkal G, et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS-CoV-2 vaccine candidates in the Syrian hamster model. iScience 2021;24:102054. doi: 10.1016/j.isci.2021.102054. Epub 9 Jan 2021 (accessed on 10 Jan 2021). [Google Scholar] |
| 3. | Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. Epub 15 Oct 2020. [Google Scholar] |
| 4. | Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30843-4. Epub 17 Nov 2020. [Google Scholar] |
| 5. | ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04619628. Safety and immunogenicity of COVI-VAC, a live attenuated vaccine against COVID-19; 2020 Nov 6. Available at https:// clinicaltrials.gov/ct2/show/NCT04619628 (accessed on 7 Jan 2021). [Google Scholar] |
| 6. | Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020;383:2320–32. [Google Scholar] |
| 7. | New York Times. Covid-19 vaccine tracker. Available at www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 7 Jan 2021). [Google Scholar] |
| 8. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet doi: 10.1016/S0140-6736(20)32661-1. Epub 8 Dec 2020 (accessed on 7 Jan 2021). [Google Scholar] |
| 9. | Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Science 1992;255:996–8. [Google Scholar] |
| 10. | Sandbrink JB, Shattock RJ. RNA vaccines: A suitable platform for tackling emerging pandemics? Front Immunol 2020;11:608460. [Google Scholar] |
| 11. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [Google Scholar] |
| 12. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med doi: 10.1056/NEJMoa2035389. Epub 30 Dec 2020 (accessed on 10 Jan 2021). [Google Scholar] |
Fulltext Views
3,551
PDF downloads
421




