Translate this page into:
Right ventricular dysfunction in rheumatic heart valve disease: A clinicopathological evaluation
2 Department of Anaesthesiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
3 Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
4 Department of Radiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
Corresponding Author:
Shantanu Pande
Department of Cardiovascular and Thoracic Surgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh
India
shantanupande@gmail.com
| How to cite this article: Pande S, Agarwal SK, Tewari P, Agrawal V, Srivastav N, Tripathi S, Soni N, Kumar S. Right ventricular dysfunction in rheumatic heart valve disease: A clinicopathological evaluation. Natl Med J India 2020;33:329-334 |
Abstract
Background. Dysfunction of the right ventricle (RV) in rheumatic heart disease (RHD) is a poor prognostic factor. We planned to observe the clinicopathological changes in the RV of patients with RHD.Methods. We defined RV dysfunction by a myocardial performance index value of >0.4 on transthoracic echo-cardiography and included patients with isolated severe mitral stenosis in sinus rhythm with normal left ventricular (LV) function from April 2014 to April 2016. The patients were divided into two groups based on the absence (group I, n=21) and presence (group II, n=22) of RV dysfunction. RV muscle biopsy was evaluated for the presence of apoptosis, fibrosis and fat deposition apart from other clinical and echocardiography parameters.
Results. Patients in both the groups had a similar demographic profile and LV dimensions and function. The age of the patients in the two groups was the only clinical parameter that was significantly different; older patients were in group II. A higher value for RV systolic pressure (RVSP) and the grade of tricuspid regurgitation was seen in group II. Though there was no significant difference in the presence of fibrosis and intensity of apoptosis in the RV biopsy samples, the deposition of fat in the interstitial spaces was decreased in group II. Age at presentation had no significant difference or correlation with the deposition of fibrosis or fat in the RV myocardial biopsy.
Conclusions. Patients with RV dysfunction were older in age and their RVSP was raised at operation, suggesting that earlier intervention may help in preserving RV function.
Introduction
Dysfunction of the right ventricle (RV) is a poor prognostic factor in long-term follow-up of patients undergoing replacement of a heart valve.[1],[2] Pulmonary artery hypertension (PAH) is one of the easily accessible clinical parameters that has a direct influence on RV function. This effect is due to increased afterload against which the RV has to perform.[3],[4] While elaborating the role of pulmonary artery pressure in a previous study, we observed that RV dysfunction was conspicuous even in patients with lower pulmonary artery pressure.[5] This indicates early appearance of RV dysfunction in rheumatic heart valve disease even before the pulmonary artery pressure starts rising. This finding initiated a study to identify factors other than raised pulmonary artery pressure that could possibly lead to RV dysfunction. One of them is fibrosis in the myocardium of patients with rheumatic heart valve disease.[6] The presence of fibrous tissue in the myocardium can affect ventricular function. Similarly, the presence of apoptosis is an unusual finding in the myocardial tissue and has been shown in the clinicopathological setting of myocardial infarction, which affects ventricular function.[7] We have also documented the upregulation of pro-apoptotic genes such as Bax and Bcl2 in patients with rheumatic heart valve disease. Apart from these factors that might affect RV function, some other factors may directly influence RV function, such as the function of the left ventricle (LV) and septum, evident as ventricular interdependence.[8] Thus, for evaluating RV function in isolation, it is important to exclude the known factors that can indirectly influence its function. In this study, we observed the effect of factors that may directly affect RV function such as raised pulmonary artery pressure, myocardial fibrosis, deposition of fat in the myocardium and apoptosis in the clinical setting of rheumatic heart valve disease with normal size and function of the LV.
Methods
We did this prospective study from April 2014 to April 2016 to assess the factors that directly influence RV function in patients with rheumatic heart valve disease. The research and ethics committee of the institute approved this study and informed consent was obtained from the patients. From April 2014 to September 2015, we recruited patients awaiting surgery for mitral valve replacement who had advanced and severe mitral stenosis. The leaflets of the mitral valves were thick, deformed and calcified. Hence, open mitral valvuloplasty was not considered. Patients with isolated severe mitral valve stenosis in normal sinus rhythm and normal LV dimensions and function were included in this study. Patients with atrial fibrillation, dilated LV with depressed function and age below 18 years and above 60 years were excluded from the study. These exclusion criteria were supposed to eliminate the effect of indirect factors affecting the RV function. Forty-three of the 124 patients requiring isolated mitral valve replacement for rheumatic heart valve disease during this period met the inclusion criteria and were finally recruited in the study. These patients underwent preoperative transthoracic echocardiographic evaluation and were divided into two groups based on the absence (group I, n=21) and presence (group II, n=22) of RV dysfunction. Clinical examination, routine investigations and echocardiographic evaluation were done in the same admission at least 3 days before the operation.
Echocardiography
Transthoracic two-dimensional (2D) echocardiography and M-mode echocardiography were done with Philips HD machine and a 3.2-MHz transducer (Philips Medical Systems, Andover, MA, USA). A single operator recorded all calculations to eliminate the bias. Patients were evaluated for size of LV end-diastolic and LV end-systolic index (LVED and LVES) and LV function by assessing LV ejection fraction (LVEF). Mitral valve assessment was done in 2D and Doppler mode for the assessment of orifice area, peak and mean gradient through the valve. Tricuspid valve regurgitation (TR) was assessed from grade I–IV, with grade I being trivial and grade IV being severe. RV systolic pressure (RVSP) was calculated in apical four-chamber view using Bernoulli’s equation (TR jet velocity) 2×4+mean right atrial pressure. RVSP was considered as representative of pulmonary artery pressure. RV function was assessed with the Doppler index of myocardial performance index (MPI) expressed by formula (isovolumic contraction time+isovolumic relaxation time/RV ejection time). Doppler probe was placed in the RV outflow tract in short-axis (SA) view to calculate the time between a and e waves (a) and ejection time (b). MPI was calculated by the following formula: MPI=a–b/b. It has been established that MPI is unaffected by heart rate, loading conditions or the presence and severity of TR. An MPI value of 0.40 and above is considered as RV dysfunction.[9] Though RV fractional area change, tricuspid annular plane systolic excursion and S velocity of all the patients were measured preoperatively apart from RVMPI in the study; only RVMPI was observed to correlate with RVEF on magnetic resonance imaging (MRI), and hence was considered for deciding two groups in the study.
Calculation of RVEF by MRI
Steady-state acquisition imaging sequences (TE 1.5 ms, TR 2.8–4.0 ms, flip angle 40°, slice thickness 8 mm and number of slices 8–12) were used to generate bright-blood cine loops in SA perpendicular to the long axis of ventricles and transverse axis parallel to the base of the heart. Vertical long-axis and horizontal long-axis views were also acquired for the overall functional assessment of the ventricle septum and wall motion. Post-gadolinium contrast (0.1 mmol/kg intravenously) myocardial delayed enhancement sequence in axial and SA views was acquired after 10–15 minutes. Two experienced reviewers independently measured RV and LVED and LVES volumes, stroke volumes and EF on the GE Advantage workstation (v 4.4) using Report Card software (v RC4.3.1). RV and LV myocardium was also evaluated for evidence of bright areas suggestive of enhancement against a backdrop of nulled myocardium in the delayed sequence. This method was considered most representative of the RV function.[10]
Surgery and RV biopsy
Patients were operated under general anaesthesia through median sternotomy using cardiopulmonary bypass with aortic and bi-caval cannulation. The right atrium was opened obliquely after snaring the vena cavae and RV was entered through the tricuspid valve after retracting it. Partial thickness of 1 cm×1 cm of the RV free wall muscle was removed from the endocardial surface. Then, moderate hypothermia of 32 °C was achieved and tepid blood antegrade cardioplegia was used after ascending the aortic cross clamp. Mitral valve was replaced through the left atrium using bi-leaflet mechanical prosthesis. The operation was then completed in the standard fashion.[9] RV muscle was dispatched immediately to the histopathology laboratory for processing and further evaluation.
Histology
RV endomyocardial biopsy was received in normal saline for frozen section and in 10% buffered formalin for routine processing.
Assessment of fat
To assess myocardial fat deposition, oil red O stain in propylene glycol (Sigma Aldrich) was performed on 4-μm cryostat sections from fresh tissue transported in normal saline and frozen in liquid nitrogen. Fat was stained reddish orange under a light microscope. The degree of fat infiltration was scored as 0: nil; 1: <10%; 2: 11%–25%; 3: 26%–50% and 4: >50%.
Assessment of fibrosis
Myocardial interstitial fibrosis was assessed on light microscopy on routine haematoxylin and eosin stain and by Masson trichrome stain on 3-μm sections from formalin-fixed, paraffin-embedded cardiac muscle tissue. A semi-quantitative assessment of the degree of interstitial fibrosis was done and scored as 0: no fibrosis; 1: <10%; 2: 11%–25%; 3: 26%–50% and 4: >50%.[11]
Assessment of apoptosis
The presence of apoptosis in the myocardium was evaluated by immune staining for Cleaved caspase 3 (ASP175; 1:500; Cell Signaling Technology), which detects activated caspase-3. Caspase-3 is responsible for the proteolytic cleavage of many key cellular proteins. Immunohistochemistry was performed on 3-μm sections of formalin-fixed, paraffin-embedded tissue as per the manufacturer’s protocol. A polymer-based peroxidase method with diaminobenzidine (Labvision; Thermo Fisher Scientific, USA) as chromogen was used to detect the bound antibodies.
Positive reaction was seen as a brown deposit in the nucleus of the cardiomyocytes. Negative and positive controls were run simultaneously. Lymph node with follicular germinative centres was used as a positive control.
The immunohistochemical expression was evaluated using an apoptotic score and the apoptotic index. A minimum of 500 cardiomyocytes per slide were studied to count the number of nuclei showing positive staining. Apoptotic index was calculated as the absolute number of positive nuclei stained per 100 cardiomyocytes.[12],[13]
Apoptotic score was calculated by scoring the proportion of positively stained cardiomyocytes (0: none; 1: <10%; 2: 11%–25%; 3: 26%–50% and 4: >50%) and the intensity of staining (0: no staining; 1: weak; 2: moderate; 3: strong) and calculating the total immune-staining score equalling the proportion score multiplied by the intensity score. The total apoptotic score ranged from 0 to 12.
Statistical analysis
The data were expressed in median and range. Non-parametric tests were used to obtain significance while comparing variables between the groups. Mann–Whitney U-test was used to calculate significance in values of a variable in two different groups. Chi-square test was used to analyse the categorical data between the groups. All analyses were done with SPSS software version 10 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patients in both the groups were similar in demographic profile and LV dimensions and function. There was difference neither in the distribution of males and females in both the groups nor in their body surface area. The New York Heart Association class was also similar in both the groups (6 v. 10 patients in class II, 12 v. 11 patients in class III and 3 v. 1 patients in class IV in group I and group II, p=0.31). The age of patients in the two groups was different with older patients present in group II [Table - 1]. A higher value for RVSP and grade of TR was seen in group II [Table - 1]. The mitral valve area has a strong negative correlation with RVSP and TR (r=–0.473, p=0.025, and r=–0.394, p=0.004, respectively), while peak gradient across the mitral valve has a strong positive correlation with RVSP (r=0.625, p=0.0001). RVSP has a strong positive correlation with TR (r=0.513, p=0.003). These findings indicate back pressure in the pulmonary circuit due to severe mitral stenosis as the cause for elevated RVSP leading to TR; however, none of the patients had organic tricuspid valve disease.
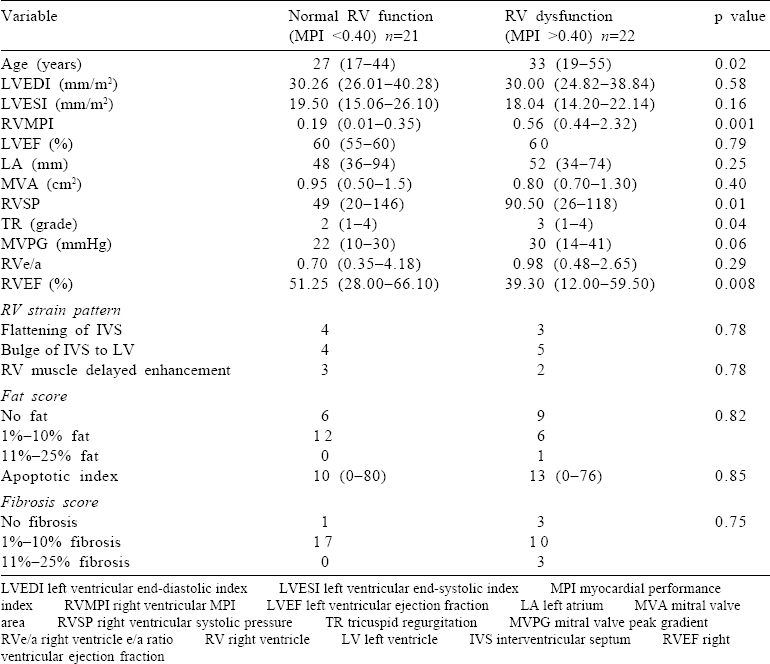
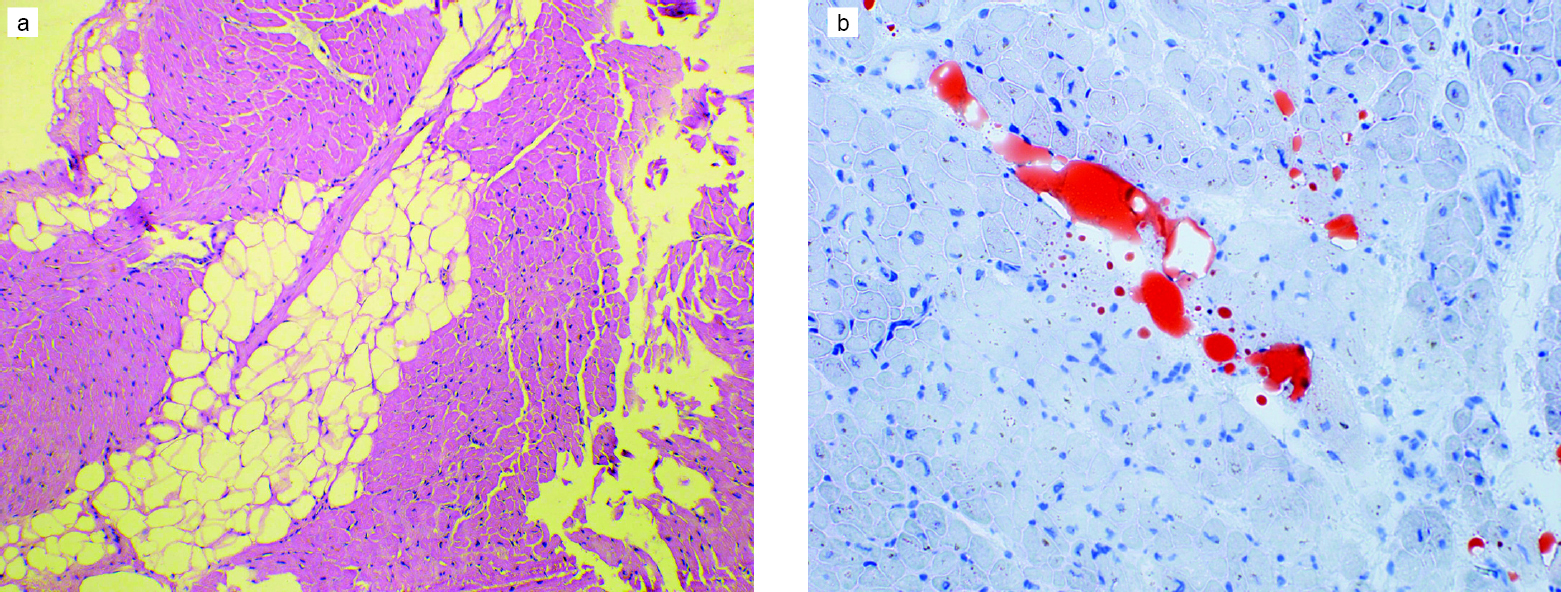
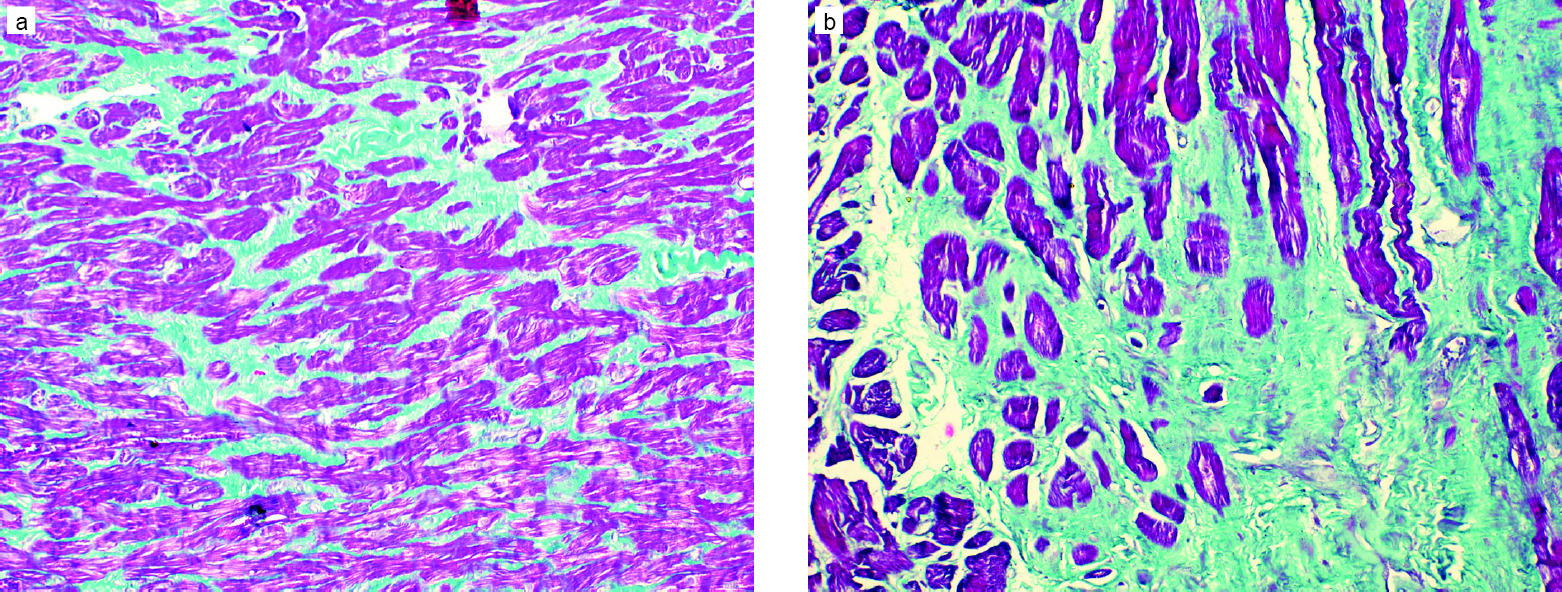
Of all the parameters of the RV studied on MRI, only EF was significantly different in the two groups, with group I showing better RV function [Table - 1]. Though there was no significant difference in the presence of fibrosis [Figure - 1] and the intensity of apoptosis [Figure - 2] in the RV biopsy sample, the deposition of fat in the interstitial spaces decreased in group II [Figure - 3]. RVMPI has a strong negative correlation with RV EF measured by MRI (r=–0.570, p=0.0001). The age of presentation and RVSP were significantly higher in group II, but were insignificant when logistic regression was done for all the factors studied. Age at presentation had no significant difference or correlation with the deposition of fibrosis in the RV myocardial biopsy. During the follow-up at 1 year, the LVED and LVES did not change significantly (p=0.32 and 0.12, respectively). There was no change in LVEF, which remained at 60% (median value), while there was a significant decrease of RVSP (77.5 mmHg [20–146] to 37 mmHg [17–102]).
 |
| Figure 1: Interstitial adipocytes in an endomyocardial biopsy: (a) comprising 11%–25% of the myocardium (H and E, ×100); (b) small amount of interstitial fat (1%–10%) highlighted by special stains (oil red O, ×200) |
 |
| Figure 2: Endomyocardial biopsy showing: (a) mild interstitial fibrosis (1%–10%) (Masson trichrome, ×200); (b) moderate interstitial fibrosis (11%–25%) (Masson trichrome, ×400) |
 |
| Figure 3: Cardiomyocytes showing moderate nuclear expression of apoptotic marker cleaved caspase-3 (active caspase-3) in an endomyocardial biopsy (diaminobenzidine, ×400) |
Discussion
We included patients with rheumatic heart valve disease involving the mitral valve and causing severe mitral stenosis. All the patients in both the groups had normal LV size and function. It is established that mitral valve stenosis is the most plausible reason for the rise of RVSP leading to the dysfunction of the RV. This finding is in agreement with our previous study on rheumatic mitral valve disease which had recruited a similar cohort of patients and established back pressure in the pulmonary circuit due to severely stenosed mitral valve orifice as the reason for the occurrence of this phenomenon.[14] Apart from raised RVSP, the age at operation was also considerably different in the two groups. It is difficult to explain why older patients in our study had more RV dysfunction. Considering that the difference in the mean age in both the groups is only 5 years, it is difficult to explain it to be an age-related change because most of the patients are in a narrow age bracket of adulthood.[15] It is interesting to observe that age at operation is not related to the deposition of fat or fibrosis and presence of apoptosis seen in the RV biopsy. Because rheumatic heart valve disease occurs in mid-teen age following repeated attacks of rheumatic fever in early childhood, the gradual decrease in the mitral valve orifice leads to a gradually rising RVSP and the older age would expose the RV to high pressures for a longer time aggravating RV dysfunction. We present this hypothesis on cause-and-effect relationship seen in this study between RVSP and RV dysfunction. We emphasize that all the patients will not have the same time-point for the onset of the proposed phenomenon.
RV dysfunction was estimated by RVMPI. MPI has been considered as the most appropriate measurement for dysfunction as it is independent of load.[16] However, this index has come under scrutiny.[17] In our study, RVMPI had a strong negative correlation with RV function estimated by MRI, and the two groups formed on values of MPI had significant differences in RVEF calculated on MRI.[18] Thus, the patients in the two groups in our study appropriately represent the basis of RV function.
Fibrosis was present in the biopsy samples from the RV myocardium from patients of both the groups, and there was no statistical difference in the quantitative estimation. The presence of fibrosis in the RV myocardium was seen in our previous study which had a similar set of patients.[9] Fibrosis in the RV myocardium could be in response to raised RVSP, but in this study, it has no relation either with the RVSP or the myocardial function measured by MPI and MRI. Since rheumatic heart disease (RHD) is an inflammatory disorder, the appearance of fibrosis can be related to inflammation. Some investigators have reported the presence of fibrosis in the myocardial samples of RHD.[19] To find the link between the appearance of fibrosis and presence of inflammatory process in patients with RHD, we have shown evidence of increase in pro-inflammatory genes in these patients.[20] The presence of fibrosis in interstitial tissue may gradually lead to depression of myocardial function, leading to cardiac failure. As we have not observed any association between myocardial function and fibrosis, it can be because we were investigating fibrosis in a piece of myocardium from free wall of the RV, and thus, the diffuse nature of fibrosis could not be established, which is a prerequisite for myocardial dysfunction.[21]
Apoptosis in the myocardium was confirmed in our study, but its effect on myocardial function could not be assessed. The presence of apoptosis in healthy myocardial cells is at the basal level.[22] Studies have observed rapid proliferation of cells followed by the process of apoptosis leading to regression of this proliferation when the stimulus is removed.[23] Most studies have investigated apoptosis in the myocardium of patients with ischaemia and reperfusion and have confirmed its association with decreased cardiac function.[24] However, few studies have observed the protective role of apoptosis in ischaemia.[25] Thus, opinion is divided on apoptosis in the heart, and this area is under intensive research. However, the presence of apoptosis in RHD has not been investigated. The appearance of apoptosis in this study establishes the finding of increased pro-apoptotic factors in Bcl-2 family proteins observed in patients with RHD.[26] There is also evidence of appearance of apoptosis in the myocardium of patients with raised afterload.[27] Thus, there is a need for further investigation on the appearance of apoptosis in patients with RHD. We also observed increase in the interstitial fat in patients with RHD. This finding deviates from the usual healthy myocardium.[28] Increase in interstitial fat content occurs in patients with increased visceral fat.[29] There are reports of glycogen deposition in myocytes during acute and convalescent myocarditis; there is no such evidence in patients with RHD.[29] This finding too needs more investigation. Few investigators have shown any shift in myocardial metabolism from free fatty acid to utilization of glucose.[30] This may be related to the findings on the deposition of interstitial fat. Except for establishing the effect of age at operation and the severity of RV pressure on the function of the RV myocardium, other factors such as fibrosis, interstitial fat deposition and apoptosis though present did not show any significant effect on RV function. This may be because these factors were investigated at the microscopic level, and the nature of their distribution in the whole myocardium could have been more informative. The other reason could be that the small number of patients investigated may not have brought out the difference in the two groups. However, these findings in RV muscle are important and need to be investigated further for their clinical significance.
Our study has a small number of patients and hence the conclusion has limited application. Though there were no considerable differences in the pathological process in the myocardial biopsy of the two groups, they are major pathological findings of the RV myocardium in patients with RHD. Another study with larger number of patients is required to elaborate on the importance of these factors on RV function in this subset of patients.
Conclusions
Our study followed the first study that based PAH as the selection criteria for creating two groups. The findings of the earlier study inspired the current one. This time, the criterion for creating groups was RV dysfunction. The haemodynamic correlation was observed as stated in many other studies, but the histological changes did not show any relation to RV dysfunction. However, the number of patients in this study being small, it is difficult to comment on the histological features that would predict RV dysfunction. Patients with rheumatic heart valve disease show evidence of inflammatory process and apoptosis in the RV. This process is ongoing even after cessation of the active rheumatic process. Patients who presented with RV dysfunction had older age at operation and had considerablly raised RVSP suggesting that earlier intervention may help in preserving the RV function.
Acknowledgements
This work was supported by a research grant from Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India (grant number: PGI/DIR/RC/161).
Conflicts of interest. None declared
| 1. | Carabello B. How to follow patients with mitral and aortic valve disease. Med Clin North Am 2015;99:739–57. [Google Scholar] |
| 2. | Jegaden O, Rossi R, Delahaye F, Montagna P, Delaye J, Delahaye JP, et al. Mitral valve replacement in severe pulmonary hypertension. Long-term results. Arch Mal Coeur Vaiss 1991;84:1297–301. [Google Scholar] |
| 3. | Jouan J, Achouh P, Besson L, Carpentier A, Fabiani JN. Advanced mitral–tricuspid disease with severe right ventricular dysfunction: The double-staged approach. Ann Thorac Surg 2012;94:992–3. [Google Scholar] |
| 4. | Vincens JJ, Temizer D, Post JR, Edmunds LH Jr., Herrmann HC. Long-term outcome of cardiac surgery in patients with mitral stenosis and severe pulmonary hypertension. Circulation 1995;92:II137–42. [Google Scholar] |
| 5. | Pande S, Agarwal SK, Dhir U, Chaudhary A, Kumar S, Agarwal V, et al. Pulmonary arterial hypertension in rheumatic mitral stenosis: Does it affect right ventricular function and outcome after mitral valve replacement? Interact Cardiovasc Thorac Surg 2009;9:421–5. [Google Scholar] |
| 6. | Sharma S, Sharma G, Hote M, Devagourou V, Kesari V, Arava S, et al. Light and electron microscopic features of surgically excised left atrial appendage in rheumatic heart disease patients with atrial fibrillation and sinus rhythm. Cardiovasc Pathol 2014;23:319–26. [Google Scholar] |
| 7. | Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA 2005;102:13807–12. [Google Scholar] |
| 8. | Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 2015;16:531–8. [Google Scholar] |
| 9. | Pande S, Tewari P, Agarwal SK, Agarwal V, Agrawal V, Chagtoo M, et al. Evidence of apoptosis in right ventricular dysfunction in rheumatic mitral valve stenosis. Indian J Med Res 2016;144:718–24. [Google Scholar] |
| 10. | Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: Accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging 2013;6:587–96. [Google Scholar] |
| 11. | Valente M, Calabrese F, Thiene G, Angelini A, Basso C, Nava A, et al. In vivo evidence of apoptosis in arrhythmogenic right ventricular cardiomyopathy. Am J Pathol 1998;152:479–84. [Google Scholar] |
| 12. | Garrity MM, Burgart LJ, Riehle DL, Hill EM, Sebo TJ, Witzig T, et al. Identifying and quantifying apoptosis: Navigating technical pitfalls. Mod Pathol 2003;16:389–94. [Google Scholar] |
| 13. | Pande S, Agarwal SK, Mohanty S, Bansal A. Effect of mitral valve replacement on reduction of left atrial size. Asian Cardiovasc Thorac Ann 2013;21:288–92. [Google Scholar] |
| 14. | Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res 2002;54:25–35. [Google Scholar] |
| 15. | Haney MF, A’Roch R, Johansson G, Poelaert J, Biber B. Beat-to-beat change in “myocardial performance index” related to load. Acta Anaesthesiol Scand 2007;51:545–52. [Google Scholar] |
| 16. | Uemura K, Kawada T, Zheng C, Li M, Shishido T, Sugimachi M, et al. Myocardial performance index is sensitive to changes in cardiac contractility, but is also affected by vascular load condition. Conf Proc IEEE Eng Med Biol Soc 2013;2013:695–8. [Google Scholar] |
| 17. | Li Y, Wang Y, Zhai Z, Guo X, Yang Y, Lu X, et al. Real-time three-dimensional echocardiography to assess right ventricle function in patients with pulmonary hypertension. PLoS One 2015;10:e0129557. [Google Scholar] |
| 18. | Fukuda K, Okada R. Histopathological studies on the myocardial fibrosis and vascular lesion of rheumatic valvular disease. Jpn Circ J 1981;45:1421–5. [Google Scholar] |
| 19. | Gupta U, Mir SS, Srivastava A, Garg N, Agarwal SK, Pande S, et al. Signal transducers and activators of transcription (STATs) gene polymorphisms related with susceptibility to rheumatic heart disease in North Indian population. Immunol Lett 2014;161:100–5. [Google Scholar] |
| 20. | Choi EY, Yoon SJ, Lim SH, Choi BW, Ha JW, Shin DH, et al. Detection of myocardial involvement of rheumatic heart disease with contrast-enhanced magnetic resonance imaging. Int J Cardiol 2006;113:e36–8. [Google Scholar] |
| 21. | Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007;13:619–24. [Google Scholar] |
| 22. | Yu P, Zhang Y, Li C, Li Y, Jiang S, Zhang X, et al. Class III PI3K-mediated prolonged activation of autophagy plays a critical role in the transition of cardiac hypertrophy to heart failure. J Cell Mol Med 2015;19:1710–19. [Google Scholar] |
| 23. | Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, et al. Urocortin inhibits beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol 2006;40:846–52. [Google Scholar] |
| 24. | Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol 1980;98:425–44. [Google Scholar] |
| 25. | Pande S, Agarwal SK, Dhir U, Chaudhary A, Kumar S, Agarwal V. Pulmonary arterial hypertension in rheumatic mitral stenosis: Does it affect right ventricular function and outcome after mitral valve replacement? Interact Cardiovasc Thorac Surg 2009;9:421–5. [Google Scholar] |
| 26. | Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, et al. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation 2008;117:3070–8. [Google Scholar] |
| 27. | Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis 2015;212:1544–51. [Google Scholar] |
| 28. | Gaborit B, Kober F, Jacquier A, Moro PJ, Cuisset T, Boullu S, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: Relationship to metabolic profile, cardiac function and visceral fat. Int J Obes (Lond) 2012;36:422–30. [Google Scholar] |
| 29. | Yu ZX, Sekiguchi M, Hiroe M, Take M, Hirosawa K. Histopathological findings of acute and convalescent myocarditis obtained by serial endomyocardial biopsy. Jpn Circ J 1984;48:1368–74. [Google Scholar] |
| 30. | Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–7. [Google Scholar] |
Fulltext Views
2,764
PDF downloads
1,254




