Translate this page into:
Young man with alleged snake bite and disseminated intravascular coagulation or a life-threatening but potentially curable malignancy: Clinicopathological correlation
Correspondence to DEEPTI MUTREJA; deeptimutreja@gmail.com
[To cite: Verma S, Baveja P, Thakur N, Patnaik S, Tilak TVSVGK, Mutreja D. Young man with alleged snake bite and disseminated intravascular coagulation or a life-threatening but potentially curable malignancy: Clinicopathological correlation. Natl Med J India 2023;36:124–8. DOI: 10.25259/NMJI_137_21]
THE CASE
A 27-year-old man, a serving soldier known to be previously healthy, was transferred to our tertiary care centre as a case of unknown bite, sepsis with disseminated intravascular coagulation (DIC) and multi-organ dysfunction syndrome (MODS) from a peripheral hospital at a remote location. There was an alleged history of redness and swelling on the left lateral aspect of the thigh following an unknown bite 3 days back. Furthermore, the patient had sustained an accidental fall due to loss of consciousness and blackish discolouration around his right eye had been documented. Investigations had revealed anaemia, mild thrombocytopenia, deranged renal and liver function tests and impaired coagulation profile. Before transfer, the patient was managed with anti-histaminics, intravenous antibiotics and intravenous fluids along with 10 units of anti-snake venom (ASV).
Clinical examination and investigations
The patient was received on day 3, and clinical examination at our centre revealed a young man, conscious, alert and obeying commands. He complained of pain in the left thigh. Pulse was 90/minutes, blood pressure (BP) 122/70 mmHg and respiratory rate was 20/minute. He had mild pallor with ecchymotic patches over the right scapula along with right periorbital and subconjunctival haemorrhage. The lateral aspect of his left thigh showed a 4×4 cm necrosed area with surrounding induration along with multiple petechiae all over his trunk and limbs. There was no evidence of compartment syndrome.
Systemic examination revealed bilateral inspiratory crackles on chest auscultation. The liver was enlarged 2 cm below the costal margin.
Investigations revealed normocytic normochromic anaemia, leucocytosis and severe thrombocytopenia. Peripheral smear findings were awaited. Prothrombin time and activated partial thromboplastin time were prolonged with a deranged international normalized ratio. His serum urea and creatinine levels were also deranged with raised liver enzymes. The arterial blood gas showed hypoxaemia with severe compensated metabolic acidosis. C-reactive protein was positive with raised titres. The biochemical investigations are summarized in Table I.
| Investigation | Results | ||
|---|---|---|---|
| Peripheral hospital | Our centre | ||
| Day 1 | Day 2 | Day 3 (0010 hour) | |
| Haemoglobin (14–18 g/dl) | 9.9 | 8.8 | 6.9 |
| Total leucocyte count (4000–11 000/μl) | 10 700 | 26 670 | 44 700 |
| Differential leucocyte count (%) | P84L12M1E3 | P95L3M2 | P87L8M3E2 |
| Platelets (150–400×103/μl) | 87 000 | 14 000 | 22 000 |
| Serum creatinine (0.6–1.3 mg/dl) | 1.83 | 3.04 | 7.13 |
| Blood urea nitrogen (7–23 mg/dl) | – | 110 | 202 |
| Bilirubin (total/direct) (mg/dl) | – | – | 1.6/0.8 |
| AST/ALT (<40 U/L) | 51.4/43.7 | 33/39 | 41/40 |
| Sodium (135–145 mmol/L) | – | – | 141 |
| Potassium (3.5–5.3 mmol/L) | – | – | 3.6 |
| Prothrombin time (11–15 seconds) (control/test) | 23.0/13 | 18.6/13 | 19/13 |
| International normalized ratio | 1.9 | 1.59 | 1.65 |
| Partial thromboplastin time (control/test) (25–35 seconds) | 41.0/30 | 49.6/30 | 29/29 |
| C-reactive protein (<5 mg/L) | 273.2 | 467 | – |
| Random blood sugar (>200 mg/dl) | 120.6 | – | 120 |
| Urine routine/microscopy | Normal | Normal | Normal |
| Wound swab culture | – | – | Sterile |
| COVID RT PCR | – | – | Negative |
An urgent chest X-ray showed diffuse fluffy opacities in the middle and lower zones of both lungs suggestive of pulmonary oedema/diffuse alveolar haemorrhage.
Course in hospital
The patient had a short stormy clinical course in our hospital. He was managed aggressively as a patient with sepsis with DIC and MODS with injectable broad-spectrum antibiotics, vitamin K and transfusion support in the form of 2 units of packed red blood cells, 4 units of fresh frozen plasma, and 6 units of random donor platelets. He was given 10 more doses of ASV keeping in mind the history of alleged unknown bite along with intravenous hydrocortisone. In view of decreased urine output and deranged kidney function tests, rescue haemodialysis was initiated. However, he showed no improvement and developed type 1 respiratory failure warranting non-invasive ventilator support. There was rapid deterioration in SpO2 from 98% to <60%, and the patient was intubated and put on mechanical ventilatory support. On direct laryngoscopy, he had copious amount of red frothy secretion in his airway and blood-stained contents were aspirated from the Ryle tube. He developed sudden onset bradycardia, hypotension and cardiac arrest, and despite all resuscitative measures, could not be revived and he finally succumbed to his illness 12 hours after admission.
TREATING UNIT’S DIAGNOSIS
DIC with sepsis and acute kidney injury
Unknown bite.
DISCUSSION
A young man, with no known comorbid conditions, presented with an ulcerated indurated swelling with central necrosis on the lateral aspect of the left thigh, following alleged unknown bite. He developed periorbital and subconjuctival haemorrhage in the right eye, following an accidental fall. Examination revealed pallor, multiple petechiae over the body, periorbital and subconjunctival haemorrhage and large area of ulceration with necrosis and induration on the lateral aspect of the left thigh.
In view of his occupation, alleged history and clinical presentation he was suspected to have a snake bite of a vasculotoxic snake belonging to Viperidae family, for which ASV was administered. Although the snake was not identified, all the clinical manifestations were akin to that of a vasculotoxic snake bite.
Snake bites are a common clinical problem in India.1 Viper envenomation is known to cause systemic toxicity in the form of coagulopathy, haemolysis, acute kidney injury (AKI), neurological complications and rhabdomyolysis.2–4 Local toxicity in the form of pain, blistering, cellulitis, abscess formation and compartment syndrome, abscess formation and gangrene are also common.4,5 Points favouring an unknown snake bite were the history suggesting the same, with a local lesion with the presence of pain at the site as well as rapid onset of systemic effects. The only point against it was the absence of a bite or fang marks at the site. Those are likely to be obviated with the onset of tissue necrosis.
The local and systemic manifestations of snake bite depend on the degree of envenomation.3,4 In our patient, general symptoms in the form of flushing, breathlessness, palpitations or tightness in chest were not reported. All the early manifestations including pain at the site, swelling, necrosis and surrounding cellulitis were present. Systemic signs of DIC and AKI were present early in this patient. DIC following snake bite can occur as early as within minutes to few hours to day’s time.6,7 Our patient also had a rapid deterioration in his condition with early onset of DIC and AKI. Coagulopathy associated with snake bite also referred to as venom-induced consumption coagulopathy causes direct endothelial damage as a result of activation of the coagulation cascade by toxins.7
Evaluation revealed bicytopenia, in the form of normocytic normochromic anaemia and thrombocytopenia with marked neutrophilic leucocytosis on the cell counter and deranged coagulation profile. In the absence of a pathologist at a remote centre, peripheral blood smear (PBS) was not studied. Fibrinogen levels, haemoglobinuria and myoglobinuria were not evaluated. Chest X-ray showed features of pulmonary oedema/diffuse alveolar haemorrhage. These findings were consistent with DIC, AKI and multi-organ failure following snake bite.
Snake venom toxins can cause damage to the nephrons, which manifests clinically with features of mild-to-severe renal impairment.4,5 Our patient did not have fever or hypotension. Fever is seen in as few as 9% and hypotension in less than 20% of patients.5,6 Late shock did occur in this patient and that is explained by hypovolaemia caused by third space losses of fluid due to increased permeability and pulmonary intravascular clotting or direct toxic effects of viper venom on the heart.5,6 Despite early administration of ASV, broad-spectrum antibiotics and haemodialysis, our patient had a fatal outcome. Factors associated with poor outcome in our patient were early onset of coagulopathy, multi-organ failure, diffuse alveolar damage and AKI.2–6
The other differential diagnosis considered was necrotizing soft-tissue infection (NSTI). NSTIs start primarily in the skin and eventually extend into the deeper tissues, namely fascia (known as necrotizing fasciitis), fat or muscle. The breach in epithelium may be caused by trauma, injections and even animal or insect bites. These are routinely caused by bacterial toxins from single or polymicrobial bacterial species and are characterized by rapid clinical disease evolution with considerable local tissue damage. Systemic manifestations depend on aetiological organisms and toxins produced.8 Necrotizing fasciitis was considered in view of the rapidly worsening symptoms. Clinically, fever is common, and local examination reveals inflamed skin, swelling and pain, further than the obvious margins of infection.9 The features against NSTI were the absence of fever and the absence of severe pain out of proportion to the wound’s appearance, pain away from the wound margins, and normal BP at admission. The wound did not appear tense enough to warrant surgical debridement. Further, NSTIs are seen more often in debilitated, diabetic, alcoholic or immunocompromised patients, our patient was a healthy young man. There are several scoring systems reported to diagnose and prognosticate NSTI as the clinical signs may sometimes be masked.10 As per these scoring criteria, our patient’s condition did not portend a favourable outcome.
FINAL CLINICAL DIAGNOSIS
Snake bite; vasculotoxic
Differential diagnosis of (a) NSTI (necrotizing fasciitis) Terminal event: DIC, diffuse alveolar damage and multi-organ failure
Comorbidity: None
PATHOLOGICAL DIAGNOSIS
Gross findings
At autopsy, the body of a young, averagely built and nourished man with multiple ecchymotic patches over back, trunk and limbs (Figs 1a and b), bilateral subconjunctival haemorrhages (Fig. 1c) and right periorbital haemorrhage were seen (Fig. 1d). Lateral aspect of the left thigh showed a large necrosed area (Fig. 1e).
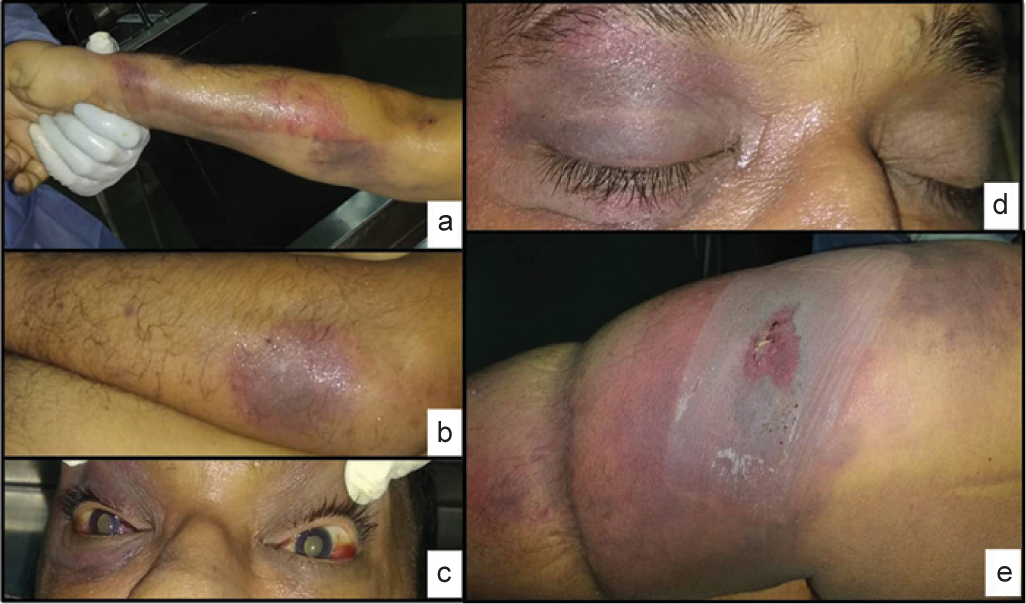
- (a and b) Upper and lower limbs, trunk showing multiple ecchymotic patches; (c) bilateral subconjunctival haemorrhage; (d) periorbital haemorrhage right eye; and (e) thigh lesion with necrosed central area and surrounding erythema
On opening the thoracic cavity, minimal blood mixed pleural fluid was present. Both left and right lungs appeared hyperaemic, were heavy, and boggy weighing 1050 g and 1200 g, respectively (Fig. 2a). Lower lobes of both lungs were congested and oozed blood-stained fluid on pressure. The heart weighed 380 g. No cardiac abnormalities, hypertrophy of ventricles or features of endocarditis were seen. The great vessels and the coronaries appeared normal. The liver was enlarged weighing 2200 g. Cut surface appeared congested (Fig. 2b). Spleen weighed 190 g and cut surface appeared beefy red (Figs 2c and d). The left and right kidneys weighed 200 g and 310 g, respectively. Capsules were non-adherent, and cut surface showed congestion with maintained corticomedullary differentiation (Fig. 2e). The brain, gastrointestinal tract, endocrine, lymphoreticular system and testes and were unremarkable on gross examination. There was minimal blood-stained peritoneal fluid. The various body fluids were collected during autopsy and analysed (Table II).

- (a) Formalin-fixed heavy and boggy right and left lungs; (b) enlarged liver with congested cut surface; (c) external surface showing enlarged spleen; (d) cut surface of spleen showing beefy appearance; and (e) formalin-fixed cut surface of the right and left kidneys showing congestion with distinct corticomedullary demarcation and normal renal pelvis
| Parameter | Cerebrospinal fluid | Pericardial fluid | Pleural fluid | Ascitic fluid | Heart blood |
|---|---|---|---|---|---|
| Appearance | Blood mixed | Clear | Blood mixed | Clear | – |
| Glucose (mg/dl) | 6 | 3 4 | 1 3 | – | 1 2 |
| Protein (mg/dl) | 5.27 | 2.7 | 3.7 | – | 7.9 |
| Albumin | – | 1.4 | 1.6 | – | 3.1 |
| LDH (i.u./L) | – | – | 4893 | – | 3 9 4 3 8 |
| Cytology | Haemorrhagic | Reactive | Benign mesothelial cells in haemorrhagic background | Benign and reactive mesothelial cells with few lymphocytes in the background | – |
| Culture | Sterile | Sterile | Sterile | Sterile | Kocuria kristinae, likely contaminant |
LDH lactate dehydrogenase
Microscopic findings
The PBS study was done after the patient was transferred to the tertiary care centre; however, the patient died soon after. The PBS was assessed postmortem and showed 68% promyelocytes with numerous cytoplasmic granules, Auer rods and faggots with few schistocytes, occasional nucleated red cells and thrombocytopenia (Fig. 3a). Strong positivity for myeloperoxidase (MPO) was seen (inset: Fig 3a).
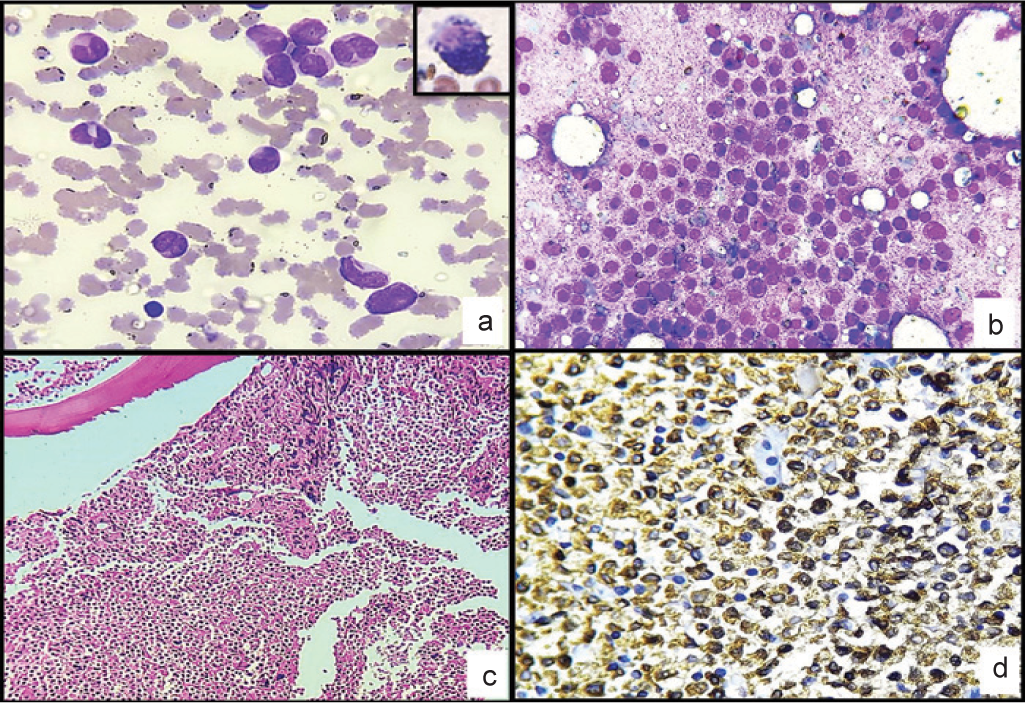
- (a) Peripheral blood. (Leishman stain; ×400) showing promyelocytes with granules, Auer rods, faggots and thromboocytopenia. (a: inset) Strong positivity for myeloperoxidase; (b) bone marrow imprint smear (Leishman stain; ×400) 90% immature myeloid cells likely promyelocytes; (c) bone marrow biopsy (H and E; ×100) 95% marrow cellularity with sheets of immature, monomorphic cells. These cells have scant cytoplasm, round nuclei and inconspicuous nucleoli; (d) (myeloperoxidase; ×400) strong myeloperoxidase-positive cells
Stained sections from the postmortem bone marrow (BM) imprint smears showed 90% immature myeloid cells with round nuclei, inconspicuous nucleoli, with moderate amount of cytoplasm filled with numerous azurophilic granules (likely promyelocytes). Mature granulocytes, erythroid precursors and megakaryocytes were markedly reduced (Fig. 3b). BM biopsy was hypercellular with approximately 95% marrow cellularity. The marrow showed diffuse infiltrate of immature, monomorphic cells with scant-to-moderate cytoplasm, round nuclei and inconspicuous nucleoli (Fig. 3c). The normal haematopoietic precursors were markedly reduced. The immature cells were strongly positive for MPO (Fig. 3d) and CD13 and negative for CD34, CD117, CD14, NSE and CD11c. CD20 was negative, while CD3 showed staining in few interspersed T cells.
Sections from both lungs showed the presence of areas of extensive intra-alveolar oedema and haemorrhage, congested blood vessels, along with thickened alveolar walls (Fig. 4a). Clusters of heart failure cells were seen. No organisms were identified. The blood vessels and interstitium showed the presence of MPO-positive leukaemic cells (inset: Fig. 4a). Sections from the liver showed normal architecture. MPO-positive leukaemic infiltrate in the portal region and hepatic sinusoids was noted (Fig. 4b).

- (H and E; ×100) (a) Lung showing extensive intra-alveolar haemorrhage, oedema, thickened alveolar membranes with leukaemic cells in blood vessels, interstitium and intra-alveolar areas; (black arrow) (b) liver shows normal architecture with leukaemic infiltrate in hepatic sinusoids and portal region; (black arrow) (c) congestion of splenic parenchyma with infiltration of leukaemic cells in the red pulp; (d) kidney showing features of acute tubular necrosis with leukaemic infiltration in the interstitium; (black arrow) leukaemic infiltrate highlighted by myeloperoxidase positivity (inset; a–d)
Sections from the spleen revealed severe congestion of splenic parenchyma with the expansion of red pulp. White pulp was attenuated with few normal lymphoid follicles. Red pulp showed infiltration by the leukaemic cells (Fig. 4c).
Sections from both kidneys showed features of acute tubular necrosis and degeneration. Leukaemic infiltration in the interstitium was noted (Fig. 4d).
Sections from the lesion in the lateral part of the left thigh showed an ulcerated area. The underlying dermis showed areas of necrosis, degeneration and oedema with congested blood vessels. Collections of neoplastic cells were seen in the epidermis, dermis and extending around dermal adnexae up to subcutis (Fig. 5a). These were strongly positive for MPO (Figs 5b and c) and CD13.
Postmortem cultures of heart blood showed the presence of a skin commensal, likely contaminant, while the ascitic and pleural fluid did not show any growth.
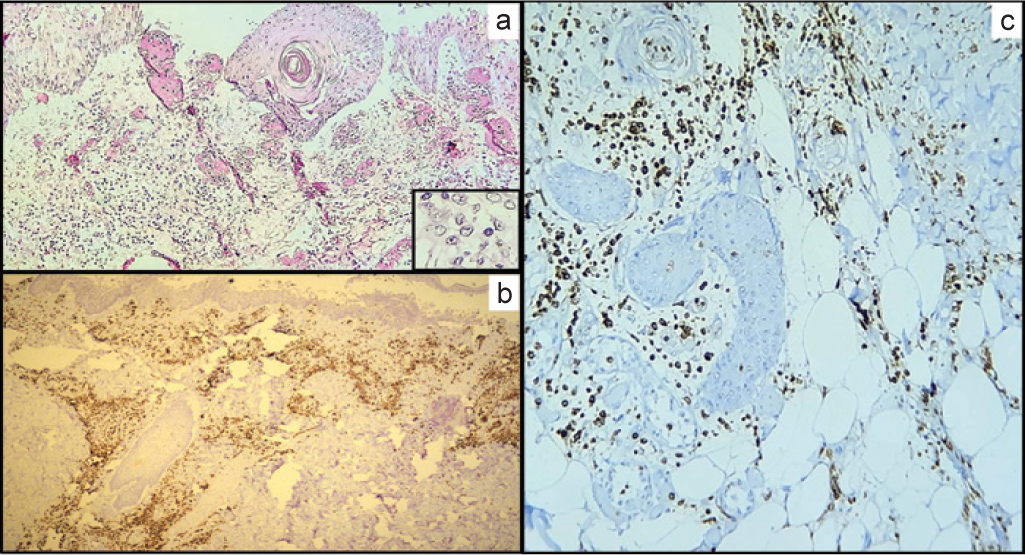
- (a) (H and E; ×100) Thigh lesion showing intact skin with ulcerated area along with collection of leukaemic cells; (highlighted in inset) (b, ×100; c, ×400) (myeloperoxidase) strong myeloperoxidase positive leukaemic infiltrate in dermis (papillary and reticular) and subcutaneous tissue of the thigh
FINAL DIAGNOSIS
Final diagnosis was acute myeloid leukaemia (AML) with DIC, sepsis and multi-MODS.
CLINICOPATHOLOGICAL CORRELATION
Postmortem findings of BM show features of AML, morphologically acute promyelocytic leukaemia (APML, French-American-British [FAB] and AML-M3), with leukaemic infiltration of various organs.
Anaemia, thrombocytopenia, bleeding manifestations and deranged coagulation profile are known features of APML. However, in the absence of cytogenetic and molecular studies, the diagnosis was presumptively made based on the morphological findings on PBS and BM imprint smears and strong MPO positivity on BM imprint smears.
The symptoms, manifestations and worsening of condition can be explained by APML. The dreaded complication of APML is DIC. The input of alleged snake bite confused the clinicians and diverted the attention towards it. APML also presents in a similar manner and is a medical emergency. The granulocytic sarcoma on the lateral aspect of the left thigh further added to the diagnostic dilemma.
Based on the clinical and autopsy findings, the diagnosis of APML (AML-M3) was made, though the confirmatory test in the form of cytogenetic or molecular studies could not be done due to non-availability of postmortem samples.
Poor prognostic factors in this patient that led to early death were DIC, multi-organ failure, thrombocytopenia, hyper-coagulability and fibrinolysis. In addition, he had anaemia and leucocytosis, all of which foretell an adverse prognosis.
Brief comment on acute promyelocytic leukemia
AML is the most common leukaemia in adults. APML is a subtype with an incidence of 7%–8% of adult AML and a median age of 47 years at presentation.11 This is the only haematological malignancy that is a medical emergency. It is essential to promptly diagnose APML based on its characteristic clinical, morphological, immunophenotypic and molecular features.11,12
As per the WHO 2016 classification, APML is classified under AML with recurrent genetic abnormalities It is also classified morphologically as AML-M3 by the FAB classification.11
Based on its morphology, APML is classified into two main types. Its major type is the classical or hypergranular variant (classical M3) that has heavily granulated cells, frequent Auer rods and faggot cells. The minor type is the hypogranular or microgranular variant (M3v), which has fine granules. These granules are not even appreciable on light microscopy giving them a dusky appearance with irregular, folded nucleus. This variant does have rare Auer rods.12,13
Immunophenotyping by flow cytometry is helpful in rapid diagnosis, though the confirmatory tests are cytogenetics, fluorescence in situ hybridization and reverse transcriptase polymerase chain reaction for PML-RARα.11–13 APML cells lack HLA-DR and CD34, while they show strong expression of myeloid markers CD33, CD13 and CD117. Myelomonocytic markers (CD11b and CD14) are negative. There was positive MPO and CD13 with negativity for CD34 in this case too. There may be aberrant expression of CD2, CD19 and CD34, which is correlated with higher white blood cell (WBC) count and M3v form.11,14
Commonly, it presents with cytopenias, has a rapid course and is associated with life-threatening coagulopathy in young adults as in this patient. With the advent of the use of all transretinoic acid (ATRA) in the past two decades, there has been a paradigm shift in the treatment of APML. ATRA along with arsenic trioxide has become the standard of care in APML. These drugs act as differentiation agents and their use in APML has transformed it into the most curable leukaemia.11,14
Unfavourable prognostic factors in APML are age >40 years at diagnosis, fever, splenomegaly and the presence of extramedullary disease.15,16 Laboratory features that indicate poor prognosis include high WBC count at diagnosis, thrombocytopenia, anaemia, deranged coagulation profile with raised D-dimer and low fibrinogen levels. Raised lactate dehydrogenase (LDH) levels, hyperuricaemia, deranged renal function and albumin levels also add to poor prognosis.11,16 At the molecular level, FLT3 mutations confer an inferior overall survival in APML. Immunophenotypic expression of CD56 and CD2 (known to be associated with higher WBC count) is another factor that can lead to fatal haemorrhage, DIC and early death.10,15,17
Extramedullary proliferation of myeloid blasts known as granulocytic sarcoma or chloroma is rare and uncommonly reported in APML.18,19 With the presentation of a granulocytic sarcoma over the left thigh and a suggestive history, a misdiagnosis of snake bite resulted in diagnostic confusion.
In clinical practice, there are no unambiguous investigative markers or tests available that may confirm snake envenomation. Affirmative recognition and identification of the incriminating snake and observation of the clinical features suggestive of envenomation only can lead to confirmation of the diagnosis.20 Thus, considering the clinical setting, a diagnosis of snake bite was not erroneous by the treating physician. However, the important diagnostic value of an early PBS cannot be undermined. Prompt and accurate diagnosis for an early intervention could have been achieved if a PBS had been done on time. Delay in diagnosis led to the dreaded complications of coagulopathy and death.
In conclusion, a clinical suspicion of APML must be considered with a rapidly progressive course of cytopenia and coagulopathy in a young adult.
Conflicts of interest.
None declared
References
- Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-epidemiological profile of snake bites over 6-year period from a rural secondary care centre of Northern India: A descriptive study. Toxicol Int. 2015;22:77-82.
- [CrossRef] [PubMed] [Google Scholar]
- Venomous and poisonous animals In: Cook GC, Zumla AI, eds. Manson's tropical diseases. Philadelphia: Saunders Elsevier; 2009. p. :557-99.
- [CrossRef] [Google Scholar]
- Snake bite-induced acute renal failure: A study of clinical profile and predictors of poor outcome. Ann Trop Med Public Health. 2012;5:335-9.
- [CrossRef] [Google Scholar]
- Clinical predictors of in-hospital mortality in patients with snake bite: A retrospective study from a rural hospital in central India. Trop Med Int Health. 2006;11:22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Snakebite doesn't cause disseminated intravascular coagulation: Coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010;36:444-51.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344-62.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing fasciitis: A deadly infection. J Eur Acad Dermatol Venereol. 2006;20:365-9.
- [CrossRef] [PubMed] [Google Scholar]
- A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000;191:227-31.
- [CrossRef] [PubMed] [Google Scholar]
- Acute myeloid leukaemia with recurrent genetic abnormalities In: Swerdlow SH, ed. WHO classification of tumors of hematopoietic and lymphoid tissues (4th ed). Lyon, France: IARC; 2017. p. :130-49.
- [Google Scholar]
- Characterization of atypical acute promyelocytic leukaemia: Three cases report and literature review. Medicine (Baltimore). 2019;98:e15537.
- [CrossRef] [PubMed] [Google Scholar]
- Acute promyelocytic leukemia: A history over 60 years-from the most malignant to the most curable form of acute leukemia. Oncol Ther. 2019;7:33-65.
- [CrossRef] [PubMed] [Google Scholar]
- Flow cytometry rapidly identifies all acute promyelocytic leukemias with high specificity independent of underlying cytogenetic abnormalities. Am J Clin Pathol. 2011;135:76-84.
- [CrossRef] [PubMed] [Google Scholar]
- Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505-15.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors in acute promyelocytic leukemia: A retrospective study of 67 cases. Leuk Lymphoma. 1991;4:249-56.
- [CrossRef] [PubMed] [Google Scholar]
- Acute promyelocytic leukemia: Clinical and morphologic features and prognostic factors. Semin Hematol. 2001;38:4-12.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation of acute promyelocytic leukemia as granulocytic sarcoma. Pediatr Blood Cancer. 2008;50:657-60.
- [CrossRef] [PubMed] [Google Scholar]




