Translate this page into:
Function-preserving surgery in sporadic desmoid-type fibromatosis of brachial plexus
Correspondence to AMOL RAHEJA; dramolraheja@gmail.com
[To cite: Dharanipathy S, Raheja A, Borkar SA, Suri A, Dutta R, Nambirajan A, et al. Function-preserving surgery in sporadic desmoid-type fibromatosis of brachial plexus. Natl Med J India 2023;36:361–3 DOI: 10.25259/NMJI_980_20]
Abstract
Desmoid tumours of the brachial plexus are rare locally infiltrative aggressive, monoclonal, fibroblastic proliferations characterized by a variable and often unpredictable clinical course. Only 21 patients have been reported in the literature. We add another one, and report function-preserving surgery in a 34-year-old man with a desmoid tumour of the brachial plexus. The patient presented with paraesthesia and gradually progressive distal muscle weakness in the left upper limb. Electrodiagnostic studies revealed preganglionic changes in segments C8–D1. Contrast-enhanced magnetic resonance imaging showed an enhancing mass with irregular margins in the left paravertebral region encasing the subclavian artery, pre- and post-ganglionic C6–D1 nerve roots and trunks of the brachial plexus. Using an anterior transclavicular approach the tumour was decompressed, which led to a major improvement in paraesthesia and partial motor recovery. He was doing well at 6 months of follow-up. Histopathological examination showed findings consistent with desmoid tumour. A tailored multidisciplinary surgical approach, with the aim to preserve function over radiological clearance, is an acceptable treatment strategy in preserving patient’s quality of life for such infiltrating desmoid tumours encasing the brachial plexus. Following surgery, observation and close radiological surveillance offer an optimal strategy without jeopardizing the quality of life.
INTRODUCTION
Brachial plexus tumours are often less amenable to total resection due to their deep location and frequent involvement of neurovascular structures. Desmoid tumours, also known as aggressive fibromatosis or desmoid-type fibromatosis are rare, locally invasive soft-tissue lesions that arise as monoclonal proliferation of mesenchymal cells in musculoaponeurotic tissues.1 Desmoids in the region of the brachial plexus are very rare and only 21 cases have been reported till recently.2–4We report another patient with a desmoid tumour involving the brachial plexus, and discuss the treatment strategy of functional preservation.
THE CASE
A 34-year-old man presented with progressive distal muscle weakness and paraesthesia in the left upper limb for 4 years. Electrodiagnostic studies revealed preganglionic changes in segments C8–D1. Imaging showed an enhancing lesion with irregular margins in the left paravertebral region. It was completely encasing the left subclavian artery, pre- and post-ganglionic segments of C6–D1 nerve roots and trunks of the brachial plexus (Figs 1a–c). We used an anterior transclavicular approach. Following clavicular osteotomy and sternoclavicular joint mobilization, control over subclavian artery and vein, vertebral artery, common carotid artery and internal jugular vein was taken (Figs 2a–d). Tumour was encountered just behind the scalenus anterior muscle and it was fibrous, highly vascular with infiltration of surrounding neurovascular structures. Intraoperative neurophysiology monitoring was used for delineation of the brachial plexus and phrenic nerve. Subtotal excision (70%–80%) was done and tumour tissue firmly adherent to the subclavian artery and lower trunk of the brachial plexus was left in situ. Brisk bleeding from the encased subclavian artery was controlled using 5-0 prolene pledgeted sutures. The clavicle was reconstructed using a dynamic compression plate and Kirschner wires. The patient had an uneventful postoperative course and the intercostal drainage tube was removed on postoperative day 5. Histopathological findings were consistent with a diagnosis of fibromatosis (Figs 3a–c). The patient had marked improvement in paraesthesia and partial improvement in motor deficits in the postoperative period, and was doing well at 6 months of follow-up. Observation and radiological surveillance (Figs 1d–f) is being used to monitor the tumour burden.
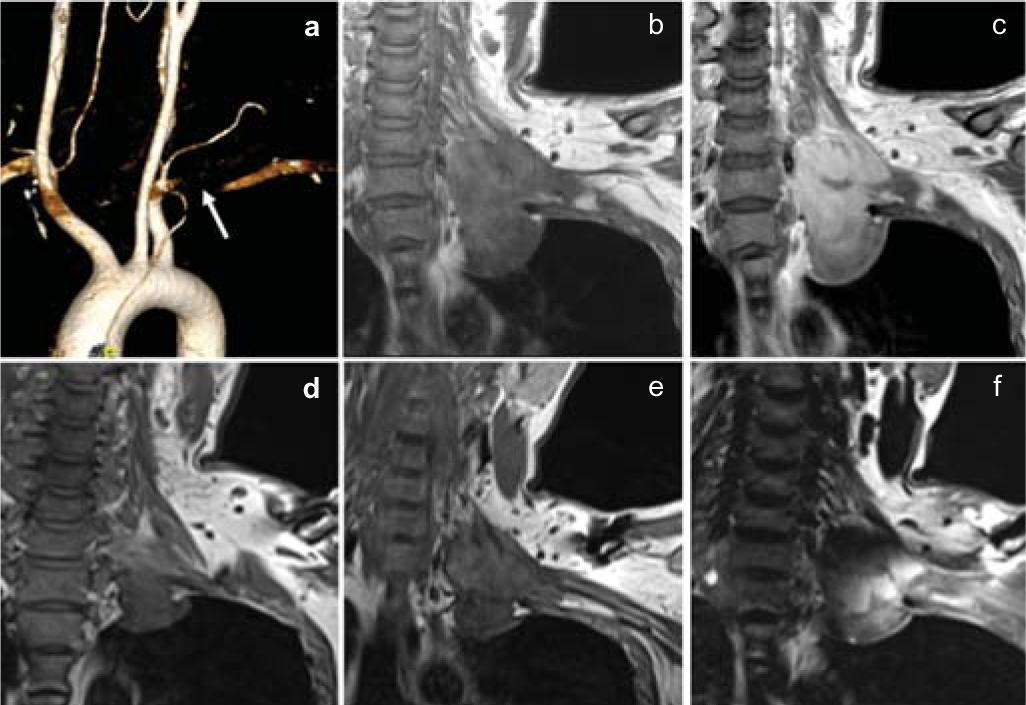
- Volume-rendering reconstruction of computed tomography images show near-total occlusion of the left subclavian artery (a). Coronal T1 (b), coronal T1-gadolinium contrast (c) magnetic resonance images show T1 isointense, heterogeneously enhancing mass lesion in the left paravertebral region extending from C6 to D2 level. Four months’ follow-up coronal T1 (d and e), coronal T1-gadolinium contrast (f) images show evidence of partial tumour decompression and residual lesion along the subclavian artery and brachial plexus in the left paravertebral region after function-preserving surgery
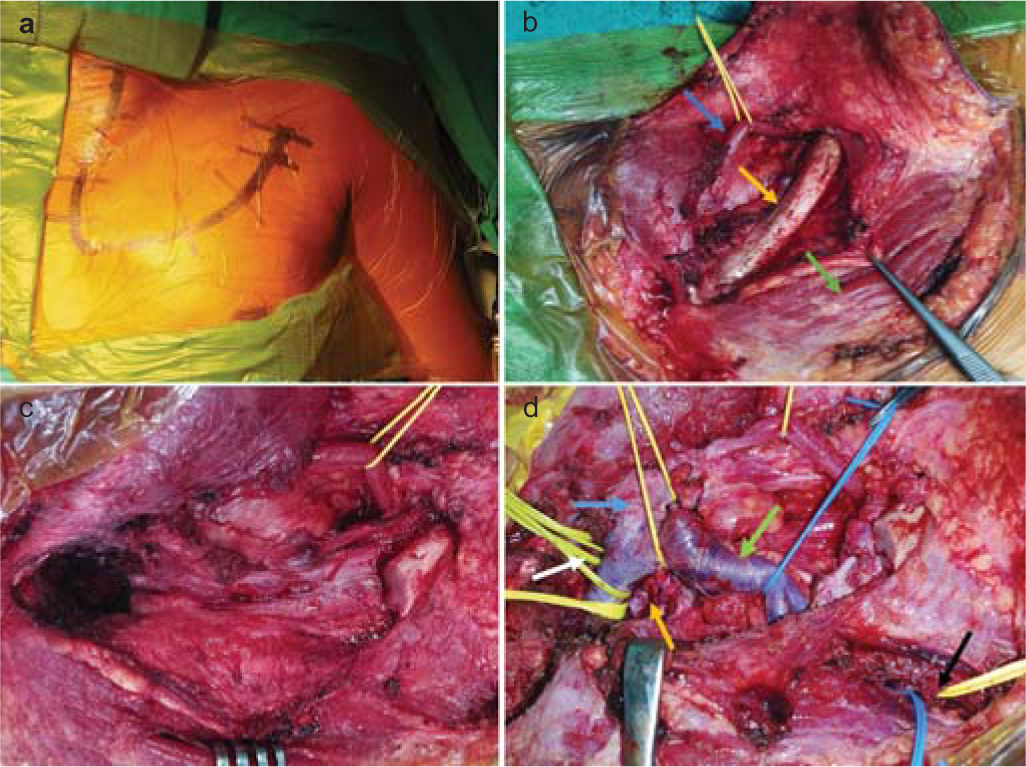
- Intraoperative images. Incision (a). Skin flap has been retracted upwards and exposed structures are shown, inferior belly of omohyoid muscle (blue arrow), clavicle (yellow arrow) and pectoralis major muscle (green arrow) (b). Clavicle at the junction of middle and lateral one-third along with sternoclavicular joint has been removed (c). Following tumour decompression, internal jugular vein (blue arrow), subclavian vein (green arrow), tumour cavity (yellow arrow), proximal (white arrow) and distal controls (black arrow) on subclavian artery (d)
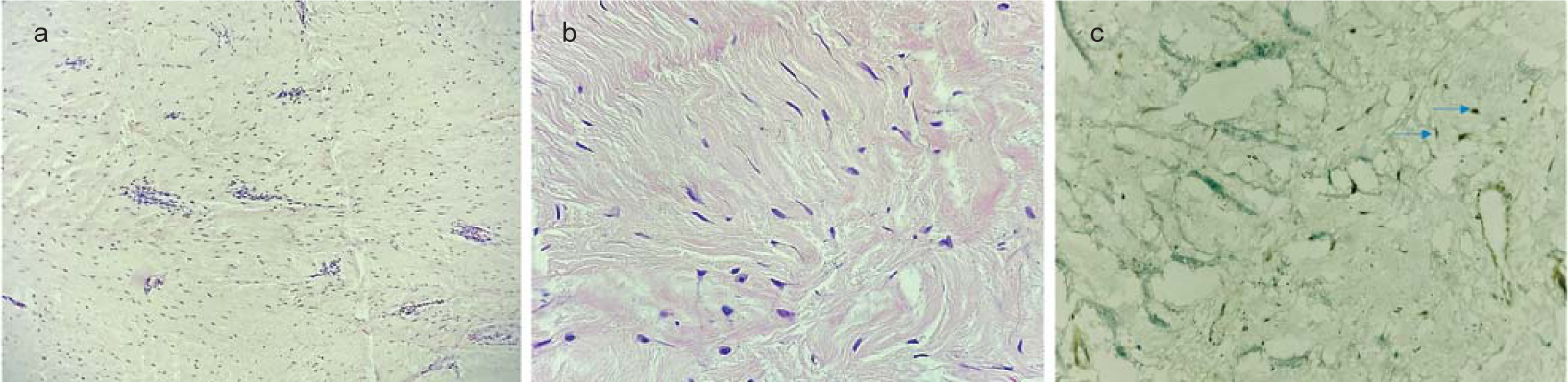
- Haematoxylin and eosin-stained sections from tumour (a ×100; b ×400) show a hypocellular spindle cell neoplasm composed of bland spindle cells in a fibrotic stroma with interspersed thin wall vessels showing perivascular lymphocytic cuffing. The tumour cells (blue arrows) show nuclear accumulation of beta-catenin on immunohistochemistry (c ×400)
DISCUSSION
Desmoid tumours account for <3% of soft tissue tumours and commonly occur in young patients (20–40 years).5 Familial forms occur commonly in the abdomen and are associated with familial adenomatosis polyposis. Sporadic forms are extra-abdominal and commonly occur in the head and neck region.6 These tumours are composed of uniformly arranged elongated spindle cells surrounded by abundant collagen fibres. Abnormal tissue repair response by fibroblast cells following blunt trauma, previous incision and even pregnancy contractions are postulated to produce fibromatosis.7 These tumours may present as a painless mass in the axilla or supraclavicular fossa, paraesthesias along the brachial plexus distribution and upper extremity weakness. Contrast-enhanced MRI is the investigation of choice. Desmoid tumours are T1 isointense, T2 hyperintense, intensely contrast-enhancing with highly infiltrative margins.8
Seinfeld et al. reported 4 patients who underwent initial gross total excision, although tumour recurrence was seen in 3 requiring a second surgery and postoperative radiotherapy. Iatrogenic hemidiaphragm paralysis occurred in 3 patients.4 Ganju et al. in 6 desmoid tumours of the brachial plexus were able to achieve complete excision in 3 patients. However, details of recurrence and radiotherapy were not available.2 Gaposchkin et al. reported 11 desmoid tumours in which 4 patients had recurrent disease and 10 patients underwent postoperative radiotherapy.3 Combining the three series and with a mean follow-up of 61 months, Seinfeld et al. found that 15 of 21 patients were disease-free and 6 patients were living with persistent disease. New onset or worsening of existing pain and weakness was also considerable (48%), highlighting the rationale of function-preserving surgery.4
We used the transclavicular approach and a team of vascular surgeons, neurosurgeons, orthopaedic surgeons and electrodiagnostic specialists were involved. Intraoperatively, we were able to demonstrate the origin of the tumour from the posterior aspect of the scalenus anterior muscle and its adjoining fascia. The tumour was highly infiltrative, attached firmly with the subclavian artery and lower trunk of the brachial plexus. Hence, we planned to proceed with function-preserving tumour decompression. Intraoperative neurophysiology monitoring helped us in early identification of the brachial plexus and phrenic nerve and their preservation. It also helped us in dissecting the tumour safely from the brachial plexus. Clavicular dissection, removal and reconstruction were done by the orthopaedic surgeons. The vascular surgeons assisted us in taking proximal and distal subclavian artery control for safe tumour decompression. Based on the current European and global consensus guidelines, observation for 1–2 years is the primary treatment strategy in operated patients.9,10 This guideline is based on the fact that upfront adjuvant radiotherapy has a high risk of transforming fibromatosis into secondary radiation-induced sarcomas, especially relevant in young patients with a long-life expectancy. In patients with life-threatening disease and tumours with aggressive behaviour, chemotherapeutic agents such as methotrexate and anthracyclines can be used. Tyrosine kinase inhibitors such as imatinib have been found to cause long-term stabilization of disease in patients with aggressive and recurrent disease.11,12 Sorafenib and pazopanib are other agents under evaluation for aggressive disease.13,14 Hence, in consultation with our medical oncologists, the plan to proceed with observation and active radiological surveillance for assessing tumour burden was taken for this patient. Chemotherapy and adjuvant radiotherapy will only be offered in a step-wise manner, if there is evidence of clinical or radiological progression of the residual tumour. This report highlights the surgical technique and role of multidisciplinary collaboration in successfully and safely managing a rare, difficult-to-treat brachial plexus desmoid tumour.
Conclusion
Desmoid tumours are rare, locally infiltrative, aggressive monoclonal, fibroblastic proliferation characterized by a variable and often unpredictable clinical course. A combined multi-disciplinary function-preserving surgical approach followed by observation and close radiological surveillance offers a reasonable treatment strategy for such tumours.
ACKNOWLEDGEMENTS
We thank Mr Namprasad and his team for assistance and technical support during intraoperative neurophysiological monitoring.
Conflicts of interest
None declared
References
- Aggressive fibromatosis(desmoid tumor) is derived from mesenchymal progenitor cells. Cancer Res. 2010;70:7690-8.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes in a consecutive series of 111 surgically treated plexal tumors: A review of the experience at the Louisiana State University Health Sciences Center. J Neurosurg. 2001;95:51-60.
- [CrossRef] [PubMed] [Google Scholar]
- Function-sparing surgery for desmoid tumors and other low-grade fibrosarcomas involving the brachial plexus. Neurosurgery. 1998;42:1297-301.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoid-type fibromatosis involving the brachial plexus. Neurosurg Focus. 2007;22:E22.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol. 2006;186:247-54.
- [CrossRef] [PubMed] [Google Scholar]
- Staging intra-abdominal desmoid tumors in familial adenomatous polyposis: A search for a uniform approach to a troubling disease. Dis Colon Rectum. 2005;48:1528-34.
- [CrossRef] [PubMed] [Google Scholar]
- A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br J Surg. 2006;93:1258-64.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging tumours of the brachial plexus. Skeletal Radiol. 2003;32:375-87.
- [CrossRef] [PubMed] [Google Scholar]
- An update on the management of sporadic desmoid-type fibromatosis: A European consensus initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG) Ann Oncol. 2017;28:2399-408.
- [CrossRef] [PubMed] [Google Scholar]
- The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. European J Cancer. 2020;127:96-10.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): An FNCLCC/French Sarcoma group phase II trial with a long-term follow-up. Ann Oncol. 2011;22:452-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumour) JCO. 2006;24:1195-203.
- [CrossRef] [PubMed] [Google Scholar]
- Pazopanib, a promising option for the treatment of aggressive fibromatosis. Anticancer Drugs. 2017;28:421-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of sorafenib in patients with desmoid-type fibromatosis. JCO. 2016;34:11065.
- [CrossRef] [Google Scholar]




