Translate this page into:
Membranous tonsillitis: Aetiology, prevalence and prescribing patterns in patients with upper respiratory tract infection
[To cite: Seçilmis Y. Membranous tonsillitis: Aetiology, prevalence and prescribing pattern in patients with upper respiratory tract infection. Natl Med J India 2024;37:69–73. DOI: 10.25259/NMJI_690_21]
Abstract
Background
Membranous tonsillitis is one of the most common forms of acute tonsillitis in childhood. Although many different microorganisms may cause this disease, clinicians generally consider bacterial agents as a possible cause and prescribe a penicillin-group of antibiotic. This study aimed to determine the aetiology of membranous tonsillitis and prescribing errors. In addition, we investigated the effectiveness of epidemiological, clinical and laboratory parameters and their role in guiding treatment.
Methods
We did this retrospective study at the paediatric emergency department of a tertiary referral hospital including 423 outpatient children aged 0 to 18 years diagnosed with membranous tonsillitis.
Results
Group A beta-haemolytic streptococcus was found in 132 (31.2%) patients, Epstein–Barr virus (EBV) in 103 (24.3%), and other viral aetiologies in 188 (44.4%). The prescription rate of antibiotics in the EBV-positive group was 27%, and Downey cells were seen at a rate of 98% in this group. Only 7% of patients with a positive throat culture were started on appropriate antibiotics.
Conclusion
EBV and group A beta-haemolytic streptococcus were the most common causes of membranous tonsillitis. Throat culture and peripheral blood smears are the most useful tests for paediatric emergency clinicians; these are fast and can help ensure correct diagnosis and guide treatment in almost all patients.
INTRODUCTION
Membranous tonsillitis presents with tonsillar inflammation, hyperaemia and swelling. The tonsil is covered with a thick grey layer and is associated with high fever and sore throat, and subsequently can involve other organ systems.1 Although many different microorganisms can cause the disease, clinicians usually consider bacterial agents as a possible cause and prescribe a penicillin-group antibiotic. However, due to aetiological differences, antibiotic-related side-effects and development of resistance, recurrent admissions to the emergency medicine department do occur.2–4
Although Corynebacterium diphtheria is still among the leading causes of membranous tonsillitis in developing countries with ineffective vaccination programmes, it is rarely seen in developed countries with high vaccination rates.5 Infectious mononucleosis is an important clinical condition that can cause membranous tonsillitis. Although this is most commonly attributed to Epstein–Barr virus (EBV), it can also be caused by other viral and some parasitic infections. Cytomegalovirus (CMV) and adenovirus, especially, may account for other factors. Streptococcal tonsillitis is also a leading bacterial agent, in addition to Corynebacterium diphtheria.6,7
The paediatric emergency physician’s approach to infection should be fast and accurate. Rapid decisions, accessible tests and appropriate treatment are the most important concerns. Thus, it is important to determine the physical examination and laboratory findings that will direct the medical team to a correct diagnosis in emergency conditions.8,9
This study aimed to determine the aetiology of membranous tonsillitis and evaluate the effectiveness of epidemiological, clinical and laboratory parameters in distinguishing it from other conditions.
METHODS
This study was approved by the institutional ethics committee (number 2021/179). Patients between 0 and 18 years of age who were admitted to our paediatric emergency department and diagnosed with membranous tonsillitis between April 2013 and April 2016 were included in the study. We conducted this retrospective study by analysing patient files and computer-automated records. Physical examination findings, laboratory parameters, treatments and clinical courses were recorded. Membranous tonsillitis was diagnosed clinically. Cases in which both tonsils were completely covered with a membrane were included in the study. Cases with no membrane and only hyperaemia or small crypts were excluded.
Patients underwent IgM testing for viral capsid antigen (VCA) by enzyme-linked immunosorbent assay. Patients with IgM antibodies against VCA for EBV infection were considered positive. In addition to membranous tonsillitis, positive VCAIgM and atypical lymphocytes in peripheral blood smear were accepted as evidence of EBV infection.
Only patients who underwent tests for EBV-VCA IgM antibody, throat culture, complete blood count, biochemistry and acute-phase reactants including C-reactive protein (CRP) levels were included in the study. Patients who underwent tests other than those specified were excluded from the study.
Inclusion criteria
Children presenting to the emergency outpatients diagnosed with membranous tonsillitis.
Exclusion criteria
Patients admitted to the hospital, those who had chronic diseases and those with upper respiratory tract infections other than membranous tonsillitis.
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA. Released 2013). The normality of the distributions of numerical variables was evaluated using the Shapiro–Wilk normality test and Q–Q graphs. Descriptive statistics are presented as number (n) and percentage (%), mean (SD) or median (minimum–maximum). Chi-square test was used to compare categorical variables. If the chi-square test revealed a difference, the Bonferroni test was used as a post-hoc test. Kruskal–Wallis analysis was used to compare variables with non-normal distributions. The Tamhane T2 test was used as a post-hoc test for the Kruskal–Wallis analysis. A p value of <0.05 was considered statistically significant.
RESULTS
A total of 423 patients aged 0–18 years were included in the study. Of these, 239 (56.5%) were boys and 184 (43.5%) were girls. The mean (SD) age of the boys was 6.22 (3.09) years and that of the girls was 6.56 (3.31) years; the overall mean (SD) age was 6.37 (3.19) years. The patients were divided into three age groups: 0–3 years, 4–10 years and over 10 years. There were 47 (11.1%) children aged 0–3 years, 308 (72.8%) aged 4–10 years, and 68 (16.1%) over 10 years of age.
Consistent with the seasonal characteristics of membranous tonsillitis, both bacterial and viral aetiologies increased in parallel in the winter months in Turkey, i.e. December and January. Although seasonal warming is observed beginning in February, the infection continued at a lower level until the middle of summer (Fig. 1).
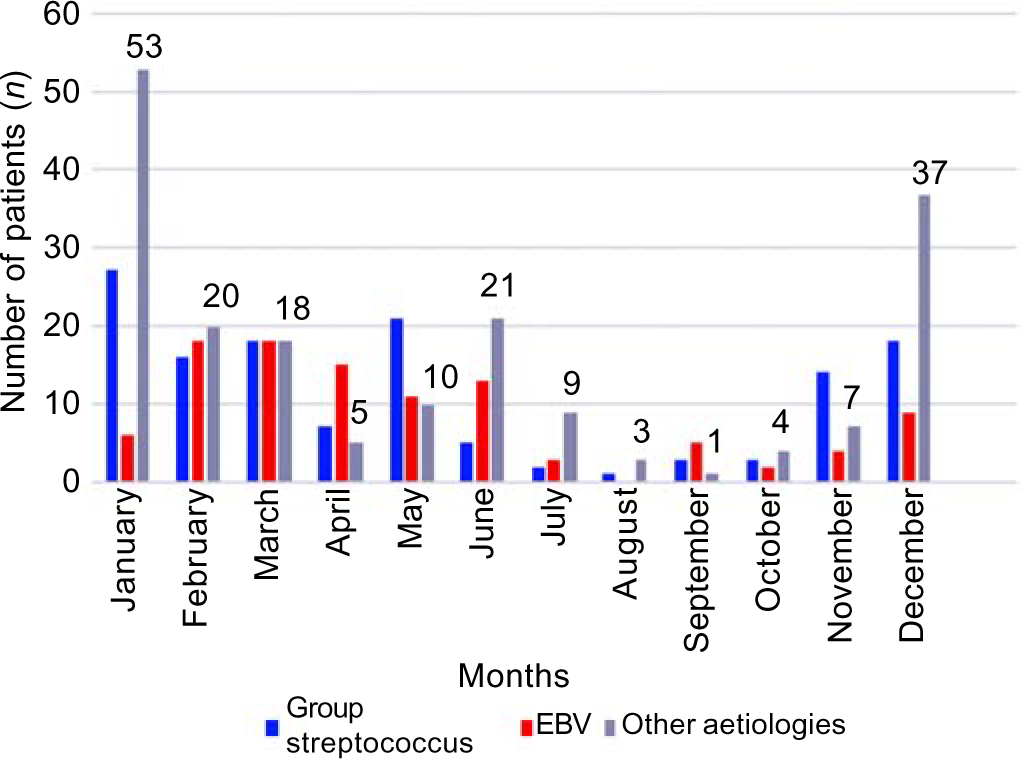
- Aetiological groups by month EBV Epstein–Barr virus
Clinical and laboratory findings
Of the patients, 293 (69.3%) had only fever, 43 (10.2%) had cough, 28 (6.6%) had sore throat, 21 (5%) had neck swelling, 17 (4.4%) had rash, 12 (2.8%) had vomiting, and 9 (2.1%) had both fever and rash. When complaints were evaluated according to age, patients in all age groups were admitted to the emergency department with similar complaints. Thus, no distinguishing clinical feature was detected by age (p>0.05).
Physical examination revealed hepatomegaly, splenomegaly, lymphadenopathy and eyelid oedema at similar frequencies in all three age groups (p>0.05). However, the evaluation of prescriptions of antibiotics showed that although viral agents were more prevalent in children <3 years old, clinicians prescribed more antibiotics (Table I).
| Item | Age groups (years) | p value | ||
|---|---|---|---|---|
| 0–3 (n=47) (%) | 4–10 (n=308) (%) | 11–18 (n=68) (%) | ||
| Median age in years (range) | 2 (1–2) | 6 (3–9) | 11 (10–16) | <0.001 |
| Boys/girls | 27/20 | 178/130 | 34/34 | 0.5 |
| Fever | 34 (72.3) | 218 (70.8) | 41 (60.3) | |
| Sore throat | 1 (2.1) | 22 (7.1) | 5 (7.4) | |
| Vomiting | 1 (2.1) | 6 (1.9) | 5 (7.4) | 0.21 |
| Rash | 2 (4.3) | 13 (4.2) | 2 (2.9) | |
| Lymphadenopathy | ||||
| Conglomerated | 1 (2.1) | 3 (1) | 2 (2.9) | 0.12 |
| Solitary | 18 (38.3) | 103 (33.4) | 21 (30.8) | |
| Hepatomegaly | 3 (6.4) | 14 (4.5) | 1 (1.5) | 0.39 |
| Splenomegaly | 2 (4.3) | 16 (5.2) | 2 (2.9) | 0.72 |
| Eyelid oedema | 1 (2.1) | 7 (2.3) | 2 (2.9) | 0.94 |
| Antibiotics prescribed (%) | 14 (29.8) | 29 (9.4) | 8 (11.8) | <0.001 |
In laboratory analyses, the incidence of streptococcal tonsillitis in the 4–10-year age group was statistically higher than that in other age groups (p=0.001). However, only 10 (7%) of 132 patients with positive throat culture were treated with antibiotics. In the 4–10-year age group, platelet levels were higher compared to other ages (p=0.01), whereas the average monocyte levels were lower (p=0.011). EBV positivity was similar in all age groups (p=0.06). No significant differences were found in other laboratory parameters (Table II).
| Item | Age groups (years) | p value | ||
|---|---|---|---|---|
| 0–3 (n=47) (%) | 4–10 (n=308) (%) | 11–18 (n=68) (%) | ||
| Median WBC (103/µl) (range) | 12.08 (3.48–28.40) | 12.66 (4.43–27.83) | 11.99 (2.100–31.00) | 0.26 |
| Mean (SD) haemoglobin (g/dl) | 12.2 (1.14) | 12.4 (1.05) | 12.7 (0.913) | 0.18 |
| Mean (SD) platelet count (103/µl) | 296.342 (105.603) | 299.642 (110.609) | 248.378 (83.123) | 0.01 |
| Median leucocyte (103/µl) (range) | 4.740 (1.45–12.20) | 3.250 (3.66–25.60) | 3.91 (5.38–18.18) | 0.4 |
| Median monocyte (%) (range) | 5.9 (2.9–10) | 5.6 (2.1–10) | 5.7 (1.7–10) | 0.01 |
| Median AST (U/L) (range) | 31 (14–600) | 22 (13–1280) | 30 (11–726) | 0.99 |
| Median ALT (U/L) (range) | 20 (9–400) | 20 (6–1280) | 20 (6–527) | 0.99 |
| Median CRP (mg/L) (range) | 5 (3–78) | 10 (3–205) | 8 (3–164) | 0.001 |
| Mean (SD) throat culture positivity | 6 (12.8) | 112 (36.4) | 14 (20.6) | 0.001 |
| VCA-IgM positivity | 14 (29.8) | 66 (21.4%) | 23 (33.8) | 0.06 |
| Atypical leucocytes (%) | 14 (29.8) | 69 (22.4) | 23 (33.8) | 0.11 |
WBC white blood cells AST aspartate aminotransferase ALT alanine aminotransferase CRP C-reactive protein VCA-IgM viral capsid antigen-immunoglobulin M
The patients were divided into three groups according to their aetiologies. The first group had 132 (31.2%) patients with positive streptococcal tonsillitis, the second group had 103 (24.3%) EBV-positive (EBV+) patients and the third group had 188 (44.4%) patients in whom other viral agents were identified. The mean age and gender were similar in all three groups (p=0.41). When evaluated in terms of clinical features, those who were EBV+ had a higher rate of sore throat, vomiting and rash than other groups. Hepatomegaly, splenomegaly and eyelid oedema were also more frequent in the EBV+ group (p<0.001). However, antibiotic prescriptions were more common in the EBV+ group than in all patients (p<0.001; Table III).
| Item | Streptococcal tonsillitis | EBV+ (n=103) (%) | Other viral aetiology | p value |
|---|---|---|---|---|
| (n=132) (%) | (n=188) (%) | |||
| Mean age in years (range) | 6 (1–14) | 6 (1–16) | 6 (1–15) | 0.41 |
| Boys/girls | 78 (59.1)/54 (40.9) | 65 (63.1)/38 (36.9) | 96 (51.1)/92 (48.9) | 0.11 |
| Fever | 110 (83.3) | 56 (54.4) | 127 (67.6) | |
| Sore throat | 8 (6.1) | 14 (13.6) | 6 (3.2) | <0.001 |
| Vomiting | 2 (1.5) | 7 (6.8) | 3 (1.6) | |
| Rash | 1 (2.3) | 12 (11.7) | 4 (2.1) | |
| Cough | 1 (0) | 3 (0) | 39 (20.7) | |
| Other | 9 (6) | 14 (13.5) | 11 (5.8) | |
| Lymphadenopathy | ||||
| Conglomerate | 0 (0) | 5 (4.9) | 1 (0.5) | <0.001 |
| Cervical | 5 6 (42) | 54 (52.4) | 3 2 (17) | |
| Submandibular | 56 (42) | 54 (52.4) | 3 2 (17) | |
| Hepatomegaly | 0 (0) | 16 (15.5) | 2 (1.1) | <0.001 |
| Splenomegaly | 1 (0.8) | 15 (14.6) | 4 (2.1) | <0.001 |
| Eyelid oedema | 0 (0) | 10 (9.7) | 0 (0) | <0.001 |
| Antibiotics prescribed (%) | 10 (7.6) | 28 (27.2) | 13 (6.9) | <0.001 |
In laboratory analyses, the mean white blood cell counts and platelet levels were significantly higher in the group with streptococcal tonsillitis (both p<0.001). Although the area under curve (AUC) value for CRP was 0.87, the values for other parameters were not significant. The highest lymphocyte ratio was found in the EBV+ group, and lymphopenia was found in the streptococcal tonsillitis group (p<0.001). The monocyte counts were similar in all three groups (p=0.96). Although the CRP level was significantly increased in the group with streptococcal tonsillitis, it was slightly higher in the EBV+ group (p<0.001). Atypical lymphocyte counts were significantly higher in the EBV+ group than in other groups (p<0.001; Table IV). The AUC value for atypical lymphocytes was high (0.981), while it was <60% for other parameters.
| Laboratory test | Streptococcal tonsillitis (n=132) | EBV (+) (n=103) | Other viral aetiology (n=188) | p value |
|---|---|---|---|---|
| Median WBC (103/µl) (range) | 16.89 (5.45–27.83) | 13.80 (2.10-31.00) | 10.02 (2.94-25.17) | <0.001 |
| Median platelet (103/µl) count (range) | a320 (104–724) | 219 (74–631) | a262 (108–410) | <0.001 |
| Median lymphocytes (103/µl) (range) | 1.719 (0.36–8.20) | 5.100 (1.00–25.60) | a4.33 (0.88–13.60) | <0.001 |
| Median CRP (mg/L) (range) | 53 (3–205) | 10 (3–164) | 3 (3–16) a | <0.001 |
| Atypical lymphocytes | 5 | 101 | 0 | <0.001 |
EBV Epstein–Barr virus WBC white blood cells CRP C-reactive protein
When EBV+ patients were evaluated according to age groups, no significant difference by age was found in terms of clinical and laboratory parameters. No distinguishing factor was found in the symptoms or physical examinations and laboratory tests at admission.
DISCUSSION
Membranous tonsillitis is seen frequently among patients admitted for upper respiratory tract infections, and a wide range of aetiological factors may be responsible for this clinical picture.2 The aetiological causes were investigated by Sharma et al. in a prospective study of 37 patients diagnosed with membranous tonsillitis in India. Corynebacterium diphtheria was found in the throat cultures of 70.3% of these patients. To a lesser extent, Streptococcus pneumonia was grown in cultures, whereas viral agents were not detected in any patient.10 Although our study included a much larger population, diphtheria was not found in any of our patients. Based on their results, Sharma et al. placed special emphasis on increasing vaccination rates.10 Due to the high vaccination rate in Turkey, Corynebacterium diphtheria was not observed in the throat culture of any patient in our study. Another bacterial pathogen, group A Streptococcus, was responsible for nearly 31% of our cases. However, only 9 of 132 cases (7%) were given appropriate antibiotic treatment.
Because viral infections may cause fever of prolonged duration, especially in children aged <3 years, children may have several visits to the emergency department. Physicians sometimes prescribe antibiotics at the first visit to avoid the need for hospitalization in the future. In addition, it is often difficult to identify the source of fever in patients aged <3 years; therefore, physicians often prescribe empirical antibiotics. The families of patients may also request for antibiotics to be given.
If the diagnosis is not clear after a detailed examination, the clinical condition of the patient and socioeconomic status of the family may guide the treatment. Patients with suspected viral infection can be re-evaluated daily, and treatment can be decided on the basis of throat culture and clinical progression.
Antibiotic prescription is based on results of throat culture after about 2 days, and these patients reported to another hospital and provided no results. The low rate of appropriate prescriptions for antibiotics may explain the high incidence of complications after group A streptococcal infections in untreated patients. In parallel, the incidence of acute rheumatic fever in Turkey is higher than that in European countries. The incidence of acute rheumatic fever in Turkey is 8.84/100 000 compared to <2/100 000 in Europe.11–13
In our study, no aetiological factor was found in many (44.4%) patients diagnosed with membranous tonsillitis. However, group A Streptococcus was grown in throat cultures from 31.2% of the remaining patients, and EBV-VCA IgM positivity was present in 24.3%. Because our study was aimed at outpatients who were examined and treated in the paediatric emergency department, more detailed viral analysis was not performed, as tests with rapid results were preferred.
The evaluation of aetiological factors according to age groups showed that the frequency of group A streptococcal infections between 3 and 10 years of age was higher than that in the other groups; the rates for those <3 years and >10 years of age were similar. A previous study in a paediatric emergency department to determine the frequency of group A streptococcal infections in patients <3 years of age found that such infections were uncommon, especially under the age of 2 years; the frequency increased slightly between 2 and 3 years of age, although the examination findings were not distinctive.14 In our study, all patients in the 0–3-year age group were under the age of 2 years at observation. However, similar to the aforementioned study, no distinguishing feature was detected on physical examination. Both studies emphasized the importance of performing throat culture in the presence of inflamed membranes or erythema, especially in patients under the age of 3 years. This is important both in terms of definitive diagnosis and to reduce inappropriate prescribing in this age group, which has the highest antibiotic prescription rate.
Although it has long been known that the CRP level is increased in patients with bacterial infections, it has also been shown that platelet levels are a good marker of bacterial infections.15–17 In our study, parameters that may increase in bacterial infections such as CRP and platelets, were significantly higher between the ages of 4 and 10 years compared to other age groups, in keeping with the fact that the frequency of bacterial infection in this age group is much more.
Analysis of the prescribing preferences of emergency physicians showed that patients in the EBV+ group were started on antibiotics at a statistically higher rate than other patients. Fever, which was the major reason for admission in this group, was observed at a similar rate in the other groups. We considered that sore throat dominates the clinical picture in these cases, occurring at a statistically higher rate than other diseases, and this led doctors to prescribe more antibiotics. In the EBV+ group, the rate of antibiotic prescription increased to 50%, especially for patients aged 0–3 years. It was observed that physical examination findings indicating infectious mononucleosis such as hepatomegaly, splenomegaly and eyelid oedema, which were significantly higher in the EBV+ group, did not affect the choice of treatment.
In a comprehensive review by Ebell et al. in 2016, sore throat was the most common symptom in EBV+ patients and detection of atypical lymphocytes in the peripheral blood smear was the most useful laboratory abnormality for diagnosis.18 Similar to previous studies, we found that sore throat was significantly more common in the EBV+ group than the other groups.
As previously noted in the literature, platelets are active cells in bacterial infections.19,20 In our study, a statistically significant elevation of platelets was detected in streptococcal tonsillitis as expected, and lymphocytosis was observed at a higher rate in the EBV+ group than in the other groups. Peripheral blood smears exhibited Downey cells in almost all (98%) EBV+ cases. These data show that the examination of peripheral blood smears is as important as throat culture in determining aetiology in patients who present with membranous tonsillitis. The AUC values for platelets in streptococcal tonsillitis and atypical lymphocytes in the EBV+ group were higher than those for the other parameters (Figs 2 and 3).
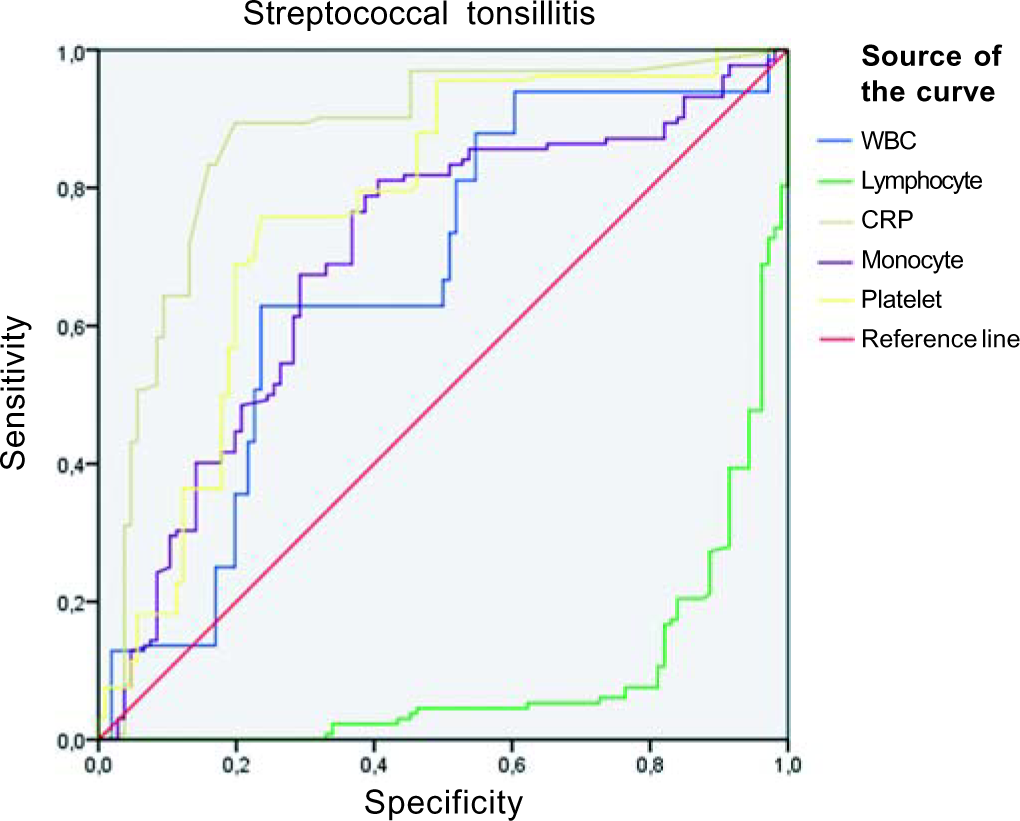
- Receiver operating characteristic curve for streptococcal tonsillitis WBC white blood cells CRP C-reactive protein
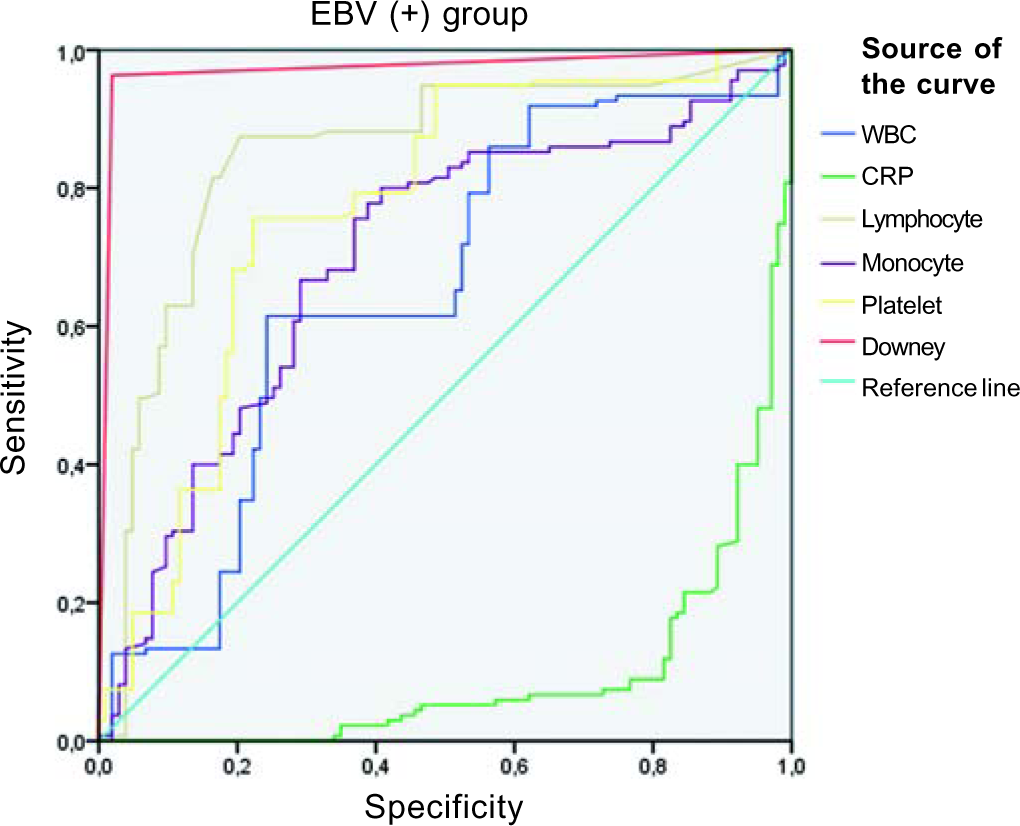
- Receiver operating characteristic curve for the Epstein–Barr virus positive (EBV+) group WBC white blood cells CRP C-reactive protein
Our study had limitations. We did not know the aetiology except in those with EBV and group A Streptococcus, although other viral causes were suspected. Due to the preference for only rapid tests in the emergency room, comprehensive analyses were not done. In our hospital, results for throat culture and EBV serology are available within 2 days, whereas viral panels take up to 15 days.
Conclusion
Our study showed that throat cultures and peripheral blood smears can be useful for the accurate diagnosis and treatment of patients with membranous tonsillitis. Although the tests and physical examination findings focus on viral infection, recurrent admission of patients due to clinically more severe fever and sore throat, and resultant parental concerns, can be attributed to clinicians’ starting the patients on antibiotics, especially in EBV+ infectious mononucleosis. A low rate of appropriate antibiotic prescription was found in positive throat culture tests.
Conflicts of interest
None declared
References
- The membranous tonsillitis during infectious mononucleosis is nevertheless of bacterial origin. Int J Pediatr Otorhinolaryngol. 1993;26:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Study of ulcero-membranous lesions of tonsil in an Indian scenario. Indian J Otolaryngol Head Neck Surg. 2017;69:16-19.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of emergency department antibiotic discharge prescription dosing errors for pediatric patients in a community hospital health system. Pediatr Emerg Care. 2020;36:e393-e396.
- [CrossRef] [PubMed] [Google Scholar]
- Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114-20.
- [CrossRef] [PubMed] [Google Scholar]
- Global epidemiology of diphtheria, 2000-2017. Emerg Infect Dis. 2019;25:1834-42.
- [CrossRef] [PubMed] [Google Scholar]
- Epstein-Barr virus In: Feigin RD, Cherry JD, eds. Textbook of pediatric infectious diseases (8th ed). Philadelphia: Elsevier; 2019. p. :1466-7.
- [Google Scholar]
- Fleisher and Ludwig's textbook of pediatric emergency medicine (8th ed). Philadelphia: Lippincott, Williams & Wilkins, USA; 2020. p. :1471-8.
- [Google Scholar]
- Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291:1587-95.
- [CrossRef] [PubMed] [Google Scholar]
- A study on acute membranous tonsillitis, its different etiologies and its clinical presentation in a tertiary referral centre. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 3):4543-8.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and clinical characteristics of acute rheumatic fever in Turkey: Results of a nationwide multicentre study. J Paediatr Child Health. 2021;57:1949-54.
- [CrossRef] [PubMed] [Google Scholar]
- Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation. 2015;131:1806-18.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15:338-40.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet toll-like receptors are crucial sensors of infectious danger moieties. Platelets. 2018;29:533-40.
- [CrossRef] [PubMed] [Google Scholar]
- The era of thromboinflammation: Platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol. 2019;10:2204.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets as key players in inflammation and infection. Curr Opin Hematol. 2020;27:34-40.
- [CrossRef] [PubMed] [Google Scholar]
- Does this patient have infectious mononucleosis?: The rational clinical examination systematic review. JAMA. 2016;315:1502-9.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets and intravascular immunity: Guardians of the vascular space during bloodstream infections and sepsis. Front Immunol. 2019;11:2400.
- [CrossRef] [PubMed] [Google Scholar]
- The role of platelets in inflammation. Thromb Haemost. 2015;114:449-58.
- [CrossRef] [PubMed] [Google Scholar]




