Translate this page into:
Anti-interleukin-6 therapies for Covid-19: A systematic review, critical appraisal and meta-analysis
2 Department of Medical Oncology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
3 Department of Clinical Haematology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
4 Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
5 Department of Pulmonary Medicine, Critical Care and Sleep Disorders, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
6 Department of Medicine, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Kameshwar Prasad
Department of Neurology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
drkameshwarprasad@gmail.com
| How to cite this article: Elavarasi A, Sahoo RK, Seth T, S, Madan K, Nischal N, Soneja M, Sharma A, Garg P, Prasad K. Anti-interleukin-6 therapies for Covid-19: A systematic review, critical appraisal and meta-analysis. Natl Med J India 2020;33:152-157 |
Abstract
Background. Coronavirus disease 2019 (Covid-19) has emerged as a pandemic by end-January 2020. Of the infected patients, 10%–15% may develop severe or critical illness. So far, no definite treatment is available for Covid-19. Cytokine release syndrome may underlie the pathogenesis of severe and critical disease. Anti-interleukin (IL)-6 therapies are being tried to improve clinical outcomes.Methods. We did a systematic review to identify the available literature on anti-IL-6 therapies in the treatment of Covid-19 and used the GRADE method to assess the quality of evidence.
Results. Four case series and 10 case reports were identified. On critical assessment, we found that these studies reported some beneficial effect of anti-IL-6 therapy, but all the studies had a high risk of bias. The pooled estimate showed that 42% of patients improved but with a very wide confidence interval (CI) (95% CI 1%–91%) and substantial heterogeneity (I2 = 95%). The overall quality of evidence was graded as ‘very low’.
Conclusions. Although promising, anti-IL-6 therapy for Covid-19 needs to be tested in randomized controlled trials to provide robust evidence.
Introduction
Coronavirus disease 2019 (Covid-19) emerged as a pandemic within two months since its initial identification in Wuhan, China, in December 2019. Though the disease runs a mild course with minimal symptoms in the majority of patients, 10%–15% of patients develop a severe or critical illness. Patients with critical illness develop respiratory failure, need intensive care and often mechanical ventilation. There is no proven effective treatment for Covid-19. The pathophysiology of the critical illness might involve cytokine release syndrome (CRS) leading to acute lung injury and high cytokine levels, particularly interleukin-6 (IL-6) levels. Further progression of the disease may be similar to secondary haemophagocytic lymphohistiocytosis causing multi-organ failure.
Initial studies have suggested that patients who develop severe illness have increased levels of cytokines, particularly IL-6 and other inflammatory mediators.[1] Pathological studies have shown that patients who died of severe disease had overexpression of Th17 cells that are stimulated by IL-6.[2],[3] The lung pathology in adult respiratory distress syndrome (ARDS) due to Covid-19 was found to be similar to interstitial pneumonia in patients with immune checkpoint inhibitor-related pulmonary toxicity.[4] Because the pathology in severe Covid-19 is akin to IL-6-related cytokine storm, there is an interest to study whether blocking IL-6 would improve clinical outcomes. Immunomodulatory agents might reduce systemic inflammation and cytokine inhibitors such as tocilizumab (TCZ), an IL-6 inhibitor, and IL-1 receptor antagonist have been used successfully in CRS triggered by other viral infections.[5] This has led to a hypothesis that immunomodulation with anti-IL-6 therapy might be associated with clinical benefits in patients with severe and critical Covid-19. IL-6 or IL-6-receptor (IL-6R) blocking antibodies such as TCZ (anti-IL-6R antibody), sarilumab (anti-IL-6R antibody) and siltuximab (anti-IL-6 antibody) are medications approved by the US Food and Drug Administration for treating various conditions, including rheumatological disease and CRS-related to chimeric antigen receptor-T cell therapy.[6] A few studies have reported the effect of anti-IL-6 therapies on the outcome of patients with Covid-19. Several clinical trials have been initiated to assess the efficacy and safety of TCZ in the management of severe Covid-19. However, because the pandemic is ongoing and the results of the current trials will take time, it is important to summarize the available data on the use of such therapies. Therefore, we did a systematic review of the available evidence on anti-IL-6 treatments for Covid-19 as well as a critical appraisal.
Methods
Criteria for considering studies for this review
We sought to identify all clinical studies including randomized controlled trials (RCTs), cohort studies, observational studies (both prospective and retrospective), case series and case reports for the purpose of this review. We also included both published and indexed studies as well as preprint and non-peer reviewed literature as Covid-19 is a new disease and the information available on these pre-print servers may be valuable in clinical practice.
We included human studies in which patients with confirmed Covid-19 of all ages and all sexes were recruited, and in which IL-6 or IL-6R blocking antibodies such as TCZ were administered in single or multiple doses.
Outcomes
For each study, we sought the following outcomes.
A Efficacy outcomes
- Clinical outcomes: clinical improvement in survival, improvement in oxygen requirements, ventilator requirements
- Radiological outcomes: improvement in findings on CT chest
- Biochemical outcomes: reduction in C-reactive protein (CRP) and change in IL-6 levels
B. Safety outcomes: adverse effects and complications associated with TCZ/siltuximab.
Search methods and identification of studies
Two authors (AE and Sh) independently searched the PubMed and Google Scholar databases using the following search string: ‘[Tocilizumab or anti-IL-6] AND [COVID-19 or SARS-CoV-2 or corona virus]’ from 2000 till 30 April 2020. No limits were applied to the search results except studies in humans. Hand searching of cross-references of original articles and reviews published or even pre-published articles was also done.
Data collection and analysis
The citations were retrieved into a reference management software (Zotero version 5.0.85) and duplicate citations were removed. All the remaining studies were reviewed on the basis of their title and abstract to select studies that met our inclusion criteria mentioned above. As all the studies were case series, the risk of bias for the studies was assessed using the Joanna Briggs Institute Tool for critical appraisal of case series. Data on outcome were extracted by one reviewer (AE) and cross-checked by another reviewer (Sh). We did a meta-analysis of the studies with 10 or more participants. The proportions of patients improved/discharged were subjected to meta-analysis using the inverse-variance method. The evidence was graded using the GRADE methodology (Grading of Recommendations, Assess-ment, Development and Evaluations).
Results
Our search yielded 45 articles on PubMed and 62 articles on Google Scholar. We included 15 from PubMed and 5 from Google Scholar for a detailed reading of the manuscript. The remaining 30 from PubMed and 57 from Google Scholar were excluded as these were not clinical studies. After excluding duplicate citations, we included 16 articles for further analysis. The Cohen’s kappa to asses inter-rater agreement was 0.86. Of these 16 articles, one article was excluded as no clinical outcome data were cited and only cytological outcomes were mentioned; a second article was excluded because of incomplete information and a third article was excluded as it had reported only the data on survivors. Thus, 13 articles were identified as relevant studies. Hand searching of references yielded one more case series. Hence, the final count of articles included in the review was 14. There were 4 case series and 10 case reports. The 4 case series were used for the meta-analysis. There were no RCTs, comparative studies or cohort studies with a comparison with historical controls. [Figure - 1] shows the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flowchart.
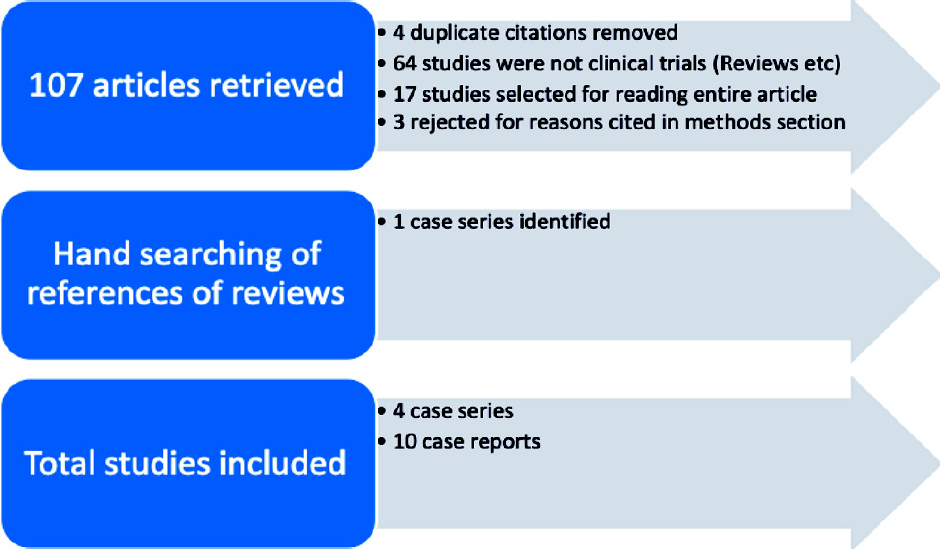 |
| Figure 1: PRISMA flow diagram. PubMed search terms: ‘(Tocilizumab or anti-IL-6) AND (COVID-19 or SARS-CoV-2 or coronavirus)’ |
Interleukin-6 and Interleukin-6 Receptor Blockade: Summary of the Selected Studies
TCZ treatment in coronavirus disease 2019: A single-centre experience[7]
In this retrospective case series with no comparator arm, a series of 15 patients (12 men, 3 women) with Covid-19 and raised IL-6 and CRP were treated with TCZ. The median age of the patients was 73 (62–80) years. The severity of the disease was moderate in 2, serious in 6 and critical in 7 patients, which was defined clinically as per the Centers for Disease Control (CDC), China criteria (Trial Version 6, Revised).[8] Ten of 15 patients had associated comorbid conditions such as diabetes, hypertension and a history of stroke (5 critical patients and 5 serious patients). All had elevated CRP (≥5 mg/L) and IL-6 (>6 pg/mL) levels. The median CRP level was 127 (10.7–258) mg/L; the median level of IL-6 was not mentioned.
Ten patients received a single dose and 5 patients received two or more doses of TCZ 80–600 mg/dose. In addition, methylprednisolone was given to 8 patients. There was no comparison group and the outcomes were assessed 1 week after the administration of TCZ. The clinical outcomes are summarized in [Table - 1] but the radiological outcomes were not described. Of the 15 patients, 9 stabilized and 1 improved. In 3 patients who died, CRP was decreased by 4–10 times, but there was no correlation with clinical outcome. In 2 patients whose condition deteriorated, 1 had a fall in CRP, and in the other patient, there was an initial fall followed by a rise. In 10 patients, the CRP levels reduced. IL-6 levels increased in 13 patients after TCZ and came down in 2 patients.
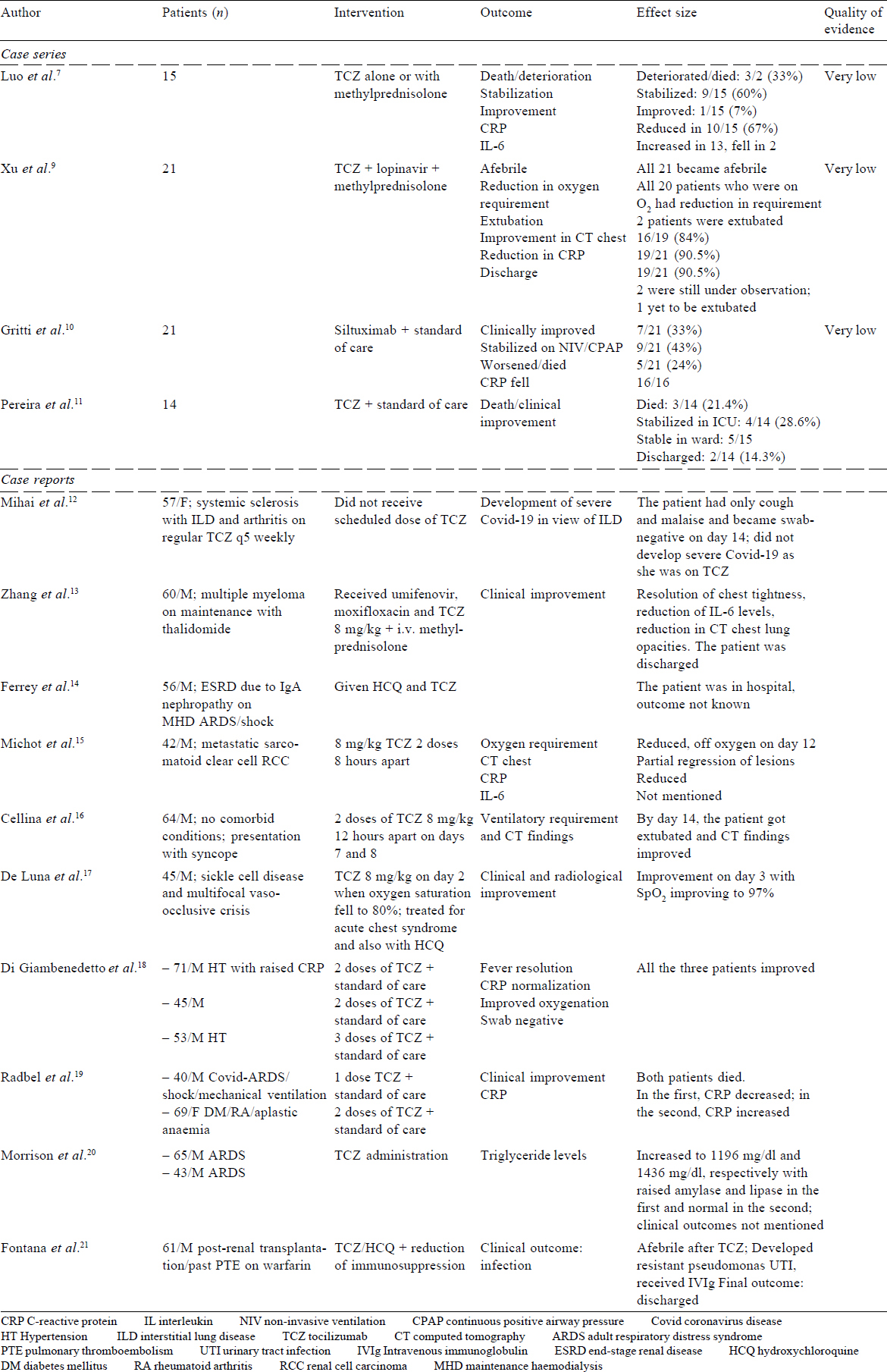
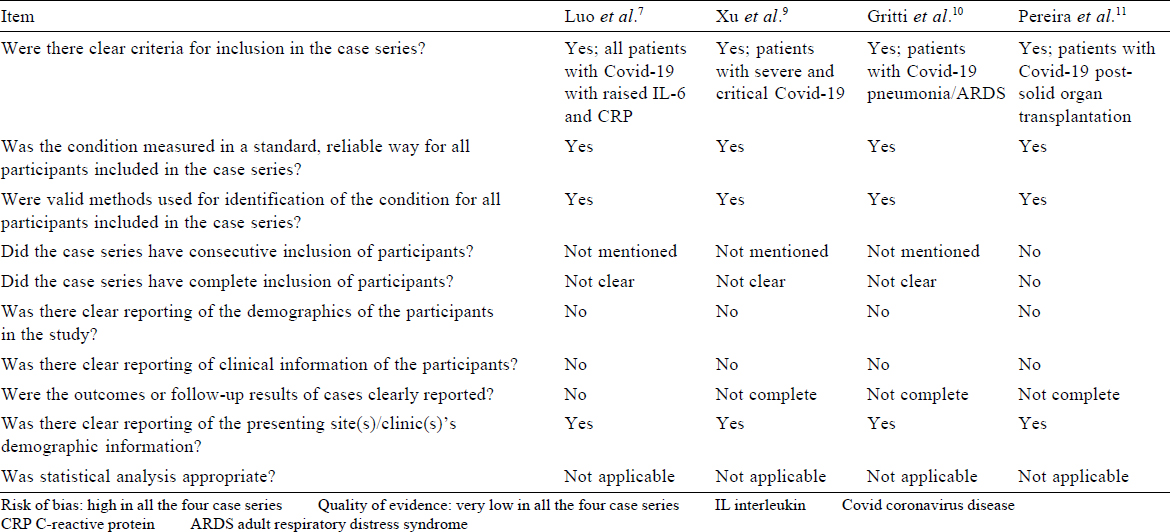
Critical appraisal. [Table - 2] shows that neither the duration of illness before administration of TCZ nor at what point of time administration of TCZ was decided were clearly mentioned. The intervention was not standardized, and varying doses were used (80–600 mg), which were not weight-based. Administration of steroids was an important confounder. More importantly, the final outcome was not known; the findings were reported as on day 7 of treatment, which was too short a period to assess clinically important outcomes. Only 1 patient had improved at the end of 1 week. The condition of the remaining patients either stabilized or deteriorated, and the final outcome was not known.
Effective treatment of severe coronavirus disease 2019 patients with TCZ[9]
This retrospective case series had no comparator arm. It included 21 (18 men, 3 women) patients with a mean age of 57 (25–88) years; 17 patients had severe and 4 had critical disease as per the CDC China criteria.[8] Hypertension (9/21) and diabetes (5/21) were the most common comorbid conditions. The duration of illness was 6 (range 2–14) days. The CRP levels were raised in all the patients (mean [SD] 75.06 (66.80) mg/L). The mean (SD) IL-6 level was 132.4 (278.5) pg/ml. A single dose of TCZ 400 mg along with lopinavir and methylprednisolone was given to 18 patients and two doses were given to 3 patients. Two patients had not been not discharged and 1 was still on a ventilator at the time of publication of the study. The mean (SD) duration of hospitalization after TCZ was 13.5 (3.1) days. There was no adverse effect of the medication.
Critical appraisal. There is no explicit mention of the selection criteria and whether consecutive patients were recruited. It was not mentioned how many patients were on mechanical ventilation. According to the authors, 4 patients were critical. There was no comparator arm. There is an important issue of confounding due to the simultaneous administration of lopinavir and methylprednisolone.
Use of siltuximab in patients with coronavirus disease 2019 pneumonia requiring ventilatory support[10]
This retrospective case series with no comparator arm included 21 Covid-19 patients with pneumonia/ARDS who required ventilator support with continuous positive airway pressure (CPAP)/non-invasive ventilation (NIV). Their median age was 64 (range 48–75) years; 18 men and 3 women. Hypertension in 42.8% (9/21), cardiovascular disease in 19% (4/21) and diabetes in 23.8% (5/21) patients were the comorbid conditions. The mean CRP level was 23.4 (9.4–43.1) mg/L and the mean IL-6 level was 139.5 (113–239) pg/ml. Sixteen patients were given a single dose and 5 patients two doses of siltuximab 11 mg/kg (700–1200 mg). It was administered within 24 hours of CPAP/NIV to 18 patients and within 48 hours of starting CPAP/NIV to 3 patients. Co-interventions such as steroids and antivirals were not mentioned. Of the 21 patients, 7 improved, 9 stabilized and 5 worsened. CRP reduced to the normal range in 16 patients in whom information was available (levels not mentioned). IL-6 levels were not measured and the radiological outcomes were not mentioned.
Critical appraisal. The nature of co-interventions used as part of ‘standard’ care was not reported. There was no comparator arm [Table - 2].
Coronavirus disease 2019 in solid organ transplant recipients[11]
This was a retrospective case series of organ transplant patients undergoing treatment for Covid-19. Of 68 admitted patients, 14 had received TCZ therapy. These patients had received other therapies too, which were not explicitly mentioned. The median age was 57 years (46–68), and most patients had comorbid conditions such as diabetes, hypertension, chronic kidney disease, active cancer and HIV, and almost all of them were on baseline immunosuppression. Three patients died, the condition of 4 deteriorated and they were still in ICU, 5 patients were stable in the medical ward and 2 had been discharged.
Critical appraisal. The nature of co-interventions used as part of ‘standard’ care was not reported. There was no comparator arm [Table - 2].
Meta-analysis
A meta-analysis of the case series summarized above was done [Figure - 2]. The outcome considered was improvement/discharge, and the effect size (ES) expressed as proportion of patients improved/discharged in each case series. The inverse of variance in each of the study’s proportion provided the weight to the study. There was substantial heterogeneity between the studies (I2=95%). The summary estimate of the proportion of patients improved/ discharged was 42% with serious imprecision (95% CI 1%–91%).
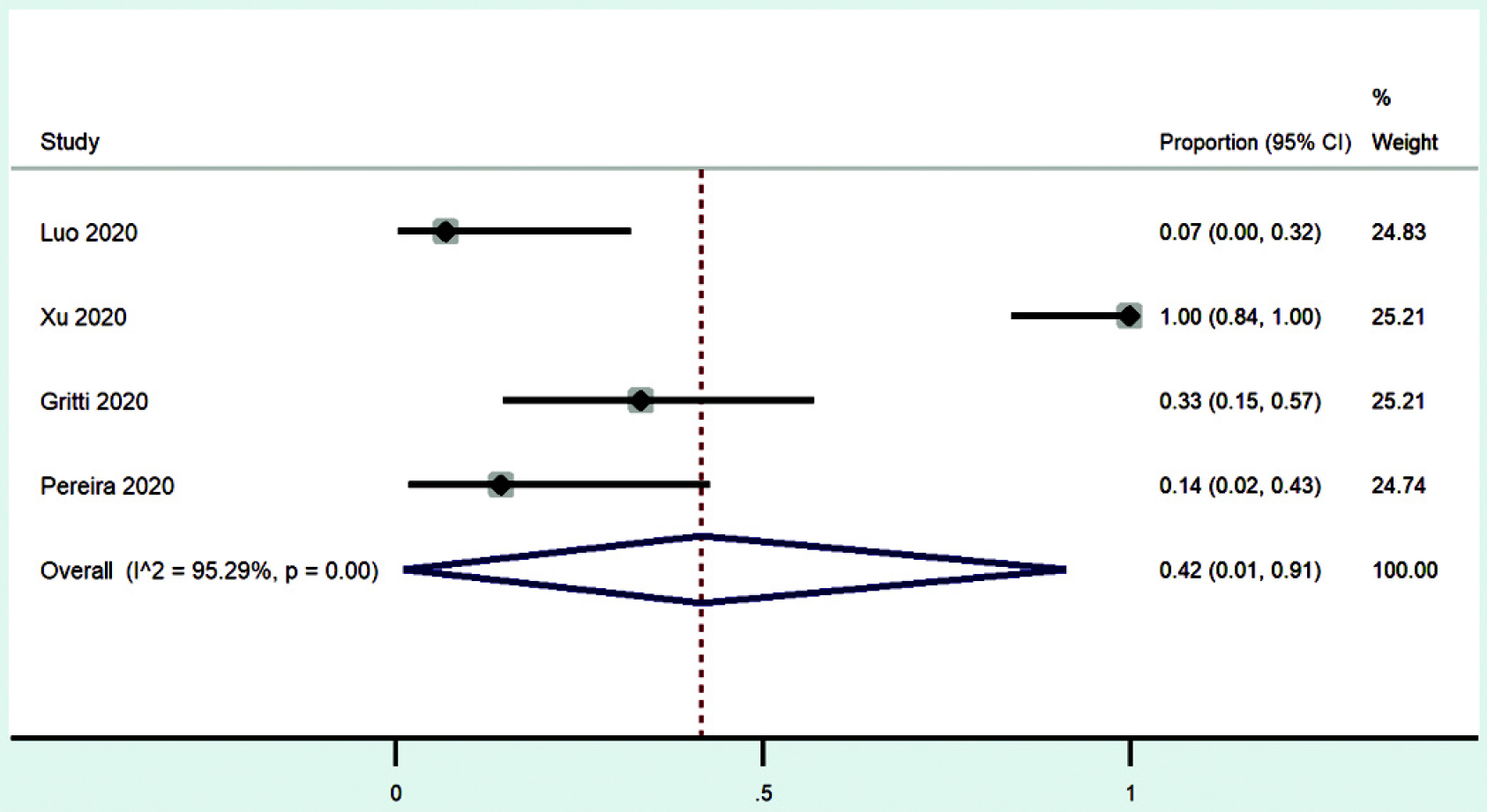 |
| Figure 2: Forest plot showing proportion of patients improved/discharged in the studies included with summary statistic |
Case reports
The critical appraisal was done using the Joanna Briggs Institute checklist for appraising case series [Table - 2].
Applying GRADE to the body of evidence
These being only observational studies, the initial quality of evidence was low. As the risk of bias was high and the sample size was small (very serious imprecision), the level of evidence was graded to be ‘very low’. The studies were not inherently designed to show the magnitude of effect, more over there was no demonstration of a dose–response gradient. Thus, the quality of evidence is ‘very low’ because it lacked the two main reasons for upgrading the quality of these studies.
Discussion
The pathophysiology of critical illness in Covid-19 might involve CRS with high cytokine levels, particularly IL-6 levels, which may lead to acute lung injury and multi-organ failure. Immunomodulatory agents might reduce systemic inflammation, and cytokine inhibitors such as TCZ have been used successfully in CRS triggered by other viral infections. Thus, there is a scientific rationale in investigating such therapy in critical Covid-19 patients.
The literature review revealed that anti-IL-6/IL-6R therapy for the treatment of severe/critical Covid-19 has been reported so far in only 4 case series and 10 case reports. The case series had significant risk of bias as none of them reported whether consecutive cases were recruited. Other key limitations were small sample size, lack of controls, moderate effect size, heterogeneity in the patient population and inconsistent results. Parameters apart from IL-6 and CRP, which were critical to the clinical decision of administering anti-IL-6 therapy such as organ failure and ARDS, were not mentioned. Another key missing information is about the cytokine storm or macrophage activation syndrome in the reported patients. Some of the case series had incomplete or inadequate follow-up data. Moreover, the three reported case series had variable effects on clinical outcome. In the case series by Luo et al.,[7] the condition of 1 of 15 (6.6%) patients improved, 5 (33.3%) worsened or died and 9 (60%) stabilized clinically at the end of 1 week after administration of TCZ. In contrast, in the series by Xu et al.,[9] the condition of all patients improved and none had secondary infections. In the study of siltuximab by Gritti et al.,[10] 24% patients died, 33% improved and were taken off mechanical ventilation and 43% were stable and continued on ventilator support till the time of the report. Pereira et al.[11] reported 21% mortality and 14% improved and fit to be discharged. The four case series seem to have incomplete outcome data, and their results were inconsistent. Overall, these case series were classified as providing very low quality of evidence. The 10 case reports recorded successful treatment of patients with comorbid conditions. However, the case reports had been published before the final outcome was known. Case reports are subject to publication bias (positive results reported more often than adverse outcomes).
A few randomized studies have been registered on ClinicalTrials.gov to assess the role of anti-IL-6 in patients with severe Covid-19. A recent report showed that anti-IL-6 therapy may not be useful in severe Covid-19 and the trial protocol has been modified to include only critical patients after review by the independent data safety monitoring board.[22] The available studies are hypothesis generating in terms of evidence and need confirmation by well-planned RCTs.
Conclusions
The evidence on the use of anti-IL-6 therapy in Covid-19 is limited. Our meta-analysis showed an improvement of 42% albeit with a wide CI of 1% to 91%, which shows very serious imprecision. In the absence of RCTs, the available evidence regarding anti-IL-6 therapy with either TCZ or siltuximab in the management of Covid-19 patients is of very low quality with a high risk of bias. Further evidence from RCTs is required to establish or refute the usefulness of these therapies in severe Covid-19.
Conflicts of interest. None declared
| 1. | Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. medRxiv 2020.03.30.20048058; [Preprint]. Available at https:// doi.org/10.1101/2020.03.30.20048058 (accessed on 22 Apr 2020). [Google Scholar] |
| 2. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020;55:105954. [Google Scholar] |
| 3. | Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 2020;18:164. [Google Scholar] |
| 4. | Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269–73. [Google Scholar] |
| 5. | Gupta L, Agarwal V, Ramanan AV. Interleukin-6 and other cytokine blockade in COVID-19 hyperinflammation. 2001. Available at www.indianjrheumatol.com/ preprintarticle.asp?id=282103 (accessed on 22 Apr 2020). [Google Scholar] |
| 6. | Buonaguro FM, Puzanov I, Ascierto PA. Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med 2020;18:165. [Google Scholar] |
| 7. | Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020;92:814–18. [Google Scholar] |
| 8. | Diagnosis and treatment protocol for novel coronavirus pneumonia (6th interim edition). China NHCOTPSRO. Available at www.kankyokansen.org/uploads/ uploads/files/jsipc/protocol_V6.pdf. (accessed on 21 April 2020). [Google Scholar] |
| 9. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020;pii:202005615. [Google Scholar] |
| 10. | Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. medRxiv 2020.04.01.20048561[Preprint] Available at https://doi.org/10.1101/ 2020.04.01.20048561 (accessed on 21 April 2020). [Google Scholar] |
| 11. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant 2020 Apr 24;10.1111/ajt.15941. doi: 10.1111/ajt.15941. Online ahead of print. [Google Scholar] |
| 12. | Mihai C, Dobrota R, Schroder M, Garaiman A, Jordan S, Becker MO, et al. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis 2020;79:668–9. [Google Scholar] |
| 13. | Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv 2020;4:1307–10. [Google Scholar] |
| 14. | Ferrey AJ, Choi G, Hanna RM, Chang Y, Tantisattamo E, Ivaturi K, et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol 2020;51:337–42. [Google Scholar] |
| 15. | Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: A case report. Ann Oncol 2020; https://doi.org/10.1016/j.annonc.2020. 03.300. pii: S0923-7534(20)36387-0. [Epub ahead of print] [Google Scholar] |
| 16. | Cellina M, Orsi M, Bombaci F, Sala M, Marino P, Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging 2020;101:323–4. [Google Scholar] |
| 17. | De Luna G, Habibi A, Deux JF, Colard M, d’Alexandry d’Orengiani ALPH, Schlemmer F, et al. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol 2020 Apr 13;10.1002/ajh.25833. doi: 10.1002/ajh.25833. Online ahead of print. [Google Scholar] |
| 18. | Di Giambenedetto S, Ciccullo A, Borghetti A, Gambassi G, Landi F, Visconti E, et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J Med Virol 2020 Apr 16. https://doi.org/10.1002/jmv.25897 [Google Scholar] |
| 19. | Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: A cautionary case report. Chest 2020. pii:S0012-3692(20)30764-9. [Google Scholar] |
| 20. | Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Letter to the Editor: Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol 2020 Apr 21. https://doi.org/10.1002/jmv.25907 [Google Scholar] |
| 21. | Fontana F, Alfano G, Mori G, Amurri A, Lorenzo T, Ballestri M, et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine. Am J Transplant 2020; 23 April. https://doi.org/10.1111/ ajt.15935 [Google Scholar] |
| 22. | Sanofi and Regeneron Provide Update on U.S. Phase 2/3 Trial in Hospitalized COVID-19 Patients. Science Business. Available at https://sciencebusiness.net/ network-updates/sanofi-and-regeneron-provide-update-us-phase-23-trial-hospitalized-covid-19. (accessed on 29 Apr 2020). [Google Scholar] |
Fulltext Views
2,304
PDF downloads
1,422




