Translate this page into:
Clinicopathological profile and outcome of adult infection-related glomerulonephritis: A prospective follow-up study
Corresponding Author:
N Gopalakrishnan
Department of Nephrology, Institute of Nephrology, Madras Medical College, Park Town, Chennai 600009, Tamil Nadu
India
srigola751@yahoo.com
| How to cite this article: Arivazhagan S, Rajasekar D, Gopalakrishnan N, Sakthirajan R, Dhanapriya J, Kumar T D, Balasubramanian T, Malathy N. Clinicopathological profile and outcome of adult infection-related glomerulonephritis: A prospective follow-up study. Natl Med J India 2020;33:260-264 |
Abstract
Background. Infection-related glomerulonephritis (IRGN) in adults is witnessing a dramatic shift in its epidemiology and outcome. Adult IRGN studies are all retrospective in nature, and Indian studies are scarce.Methods. We did this prospective study (September 2016–April 2018) on all patients with biopsy-proven IRGN and age ≥18 years satisfying three of five diagnostic criteria. Patients with persistent hypocomplementaemia (>3 months) were excluded. We did electron microscopy in those without a minimum of three diagnostic criteria and did an extensive search for any occult infection in every patient.
Results. Forty-five patients were studied with a mean (SD) follow-up of 45.7 (20) weeks. Their mean age was 41.5 years (18–70 years), with a female preponderance (1:1.25). At presentation, the majority had oedema (100%), oliguria (84.4%), hypertension (80%) and haematuria (77.8%). Of them, 86.7% had renal insufficiency and 35.6% required dialysis. Only 53.3% of them had evidence of antecedent/ current infection, with skin/subcutaneous focus being the most common site. Hypocomplementaemia was present in 82.2% of patients. Salient pathological features were endocapillary proliferation (93.3%), neutrophilic infiltration (88.9%), presence of crescents (17.8%), interstitial infiltration (24.4%), moderate-to-severe interstitial fibrosis with tubular atrophy (IFTA; 15.5%) and underlying diabetic glomerulosclerosis (8.9%). Only 66.7% of patients made complete renal recovery. By logistic regression analysis, the predictors of poor outcome were a requirement for dialysis at presentation (p=0.04) and presence of IFTA (p = 0.03).
Conclusion. A proportion of adult IRGN patients progress to chronic kidney disease.
Introduction
Infection-related glomerulonephritis (IRGN) is an immune complex-mediated acute glomerulonephritis involving both in situ and circulating immune complexes in association with a variety of non-renal infections.[1] There has been a dramatic shift in the epidemiology of IRGN over the past few decades. From being a disease typically involving children with an invariably favourable prognosis, it is increasingly becoming a disease that afflicts adults, wherein a proportion of patients progress to chronic kidney disease (CKD) and end-stage renal disease (ESRD).[2],[3],[4],[5],[6] Since the majority of adults with IRGN can have ongoing infection at the time of diagnosis, the term IRGN is preferred over post-infectious glomerulonephritis.[7]
Adult IRGN is associated with a wider spectrum of non-streptococcal agents, especially staphylococcal and Gram-negative organisms.[2],[8],[9],[10] It can be associated with a more heterogeneous set of infectious sites (beyond skin and upper respiratory tract), with few of them even harbouring infection at more than one site.[2],[9],[10] IRGN without an apparent infectious focus can be associated with occult visceral or deep-seated abscess,[10],[11],[12],[13],[14] making it imperative that there should be an extensive search for any occult primary source of infection. Aggressive measures to eradicate active infection are warranted to salvage the renal function. Adult IRGN, especially the elderly, are more likely to have underlying comorbid conditions such as diabetes mellitus, liver disease, malignancy and other immuno-compromised conditions. The presence of these has been shown to result in poor renal prognosis.[8],[9],[15] They are more likely to have severe manifestations such as dialysis-requiring renal failure, congestive heart failure, nephrotic range proteinuria and death.[9],[16]
Indian studies of adult IRGN are scarce and retrospective in nature. We did a prospective study of biopsy-proven adults with IRGN to analyse the clinicopathological profile, outcome and predictors of prognosis.
Methods
This prospective observational study was done at Madras Medical College from September 2016 to April 2018. All patients aged >18 years with a definitive diagnosis of IRGN were enrolled. As no single clinical or pathological finding is pathognomic for IRGN, the diagnosis was based on the criteria put forth by Nasr.[17] At least three of the following five criteria are required: (i) clinical or laboratory evidence of infection preceding or at the onset of glomerulonephritis; (ii) depressed serum complement; (iii) endocapillary proliferative and exudative glomerulonephritis; (iv) C3-dominant or co-dominant glomerular immunofluorescence staining; and (v) hump-shaped sub-epithelial deposits on electron microscopy (EM). Those with HIV and hepatitis B or C infection were excluded. IgA-dominant IRGN patients were excluded. Those with hypocomple-mentaemia beyond 3 months were also excluded.
History, physical examination and laboratory investigation findings were documented. The presence of underlying comorbid conditions such as diabetes mellitus, alcoholism, malignancy, immunosuppressive intake, cirrhosis, HIV seropositivity, intravenous drug abuse and severe malnutrition was noted.
All patients had the following investigations: complete blood count with peripheral smear, urine analysis; urine protein–creatinine ratio (uPCR); blood sugar; blood urea; serum creatinine; serum electrolytes; liver function tests; serum albumin; serological tests for HIV, hepatitis B and hepatitis C; complement levels (C3 and C4); antistreptolysin O titre and C-reactive protein levels. Antinuclear antibody (ANA) and antineutrophil cytoplasmic antibodies (ANCA) were done in selected patients.
Kidney biopsy was done in all patients under ultrasound guidance using the Bard Max-core disposable core biopsy instrument. Tissue samples were studied under light microscopy with haematoxylin and eosin, periodic acid–Schiff, Jone’s methena-mine silver and Masson’s trichrome stains. Immuno-fluorescence (IF) staining was performed on 3-μm cryostat sections using polyclonal fluorescein-isothiocyanate-conjugated antibodies to IgG, IgM, IgA, C3, C1q, C4, kappa and lambda light chains. The intensity of IF staining was graded on a scale of 0–3. All the biopsy samples were reported by the same renal pathologist. EM was done only in those patients in whom three of the five IRGN diagnostic criteria were not fulfilled without EM.
An extensive search for any active focus of infection was done in every patient with an emphasis not to miss any occult active infection. The sepsis screen included evaluation of the ear, nose, throat, oral cavity including teeth; dermatology evaluation; echocardiography to rule out infective endocarditis; chest X-ray; ultrasound abdomen and culture of blood, urine, pus and other appropriate samples. Treatment included eradication of any infectious focus, salt and fluid restriction, blood pressure control with antihypertensives and dialysis support, whenever indicated.
All patients were followed up, and the following parameters were recorded in the subsequent visits: blood pressure, uPCR, urine sediments and serum creatinine. C3 and C4 levels beyond 3 months of presentation were done in every patient. The estimated glomerular filtration rate was calculated at presentation and at subsequent visits using the CKD-EPI (chronic kidney disease epidemiology collaboration) formula (uses four variables: age, sex, race and serum creatinine). Patients with persistently depressed complement levels beyond 3 months were excluded from the study. Patients with a follow-up period of at least 3 months only were included for analysis. Informed written consent was obtained from the study participants, and the study was approved by the Institutional Ethics Committee.
Statistical analysis
Univariate analysis of categorical variables was done using the chi-square test. Statistical significance was assumed at p<0.05. Statistical analysis was done using MedCalc statistical software. Predictors of failure to achieve complete recovery were identified by logistic regression analysis.
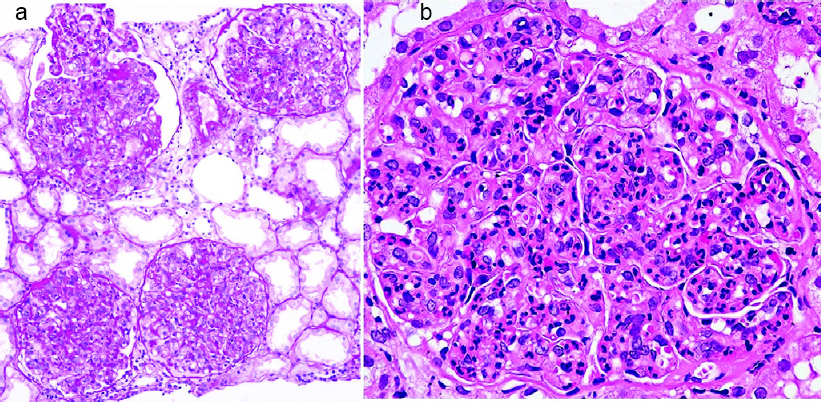 |
| Figure 1: Light microscopy (a ×10; b ×100) showing diffuse endocapillary proliferative glomerulonephritis with neutrophil infiltration |
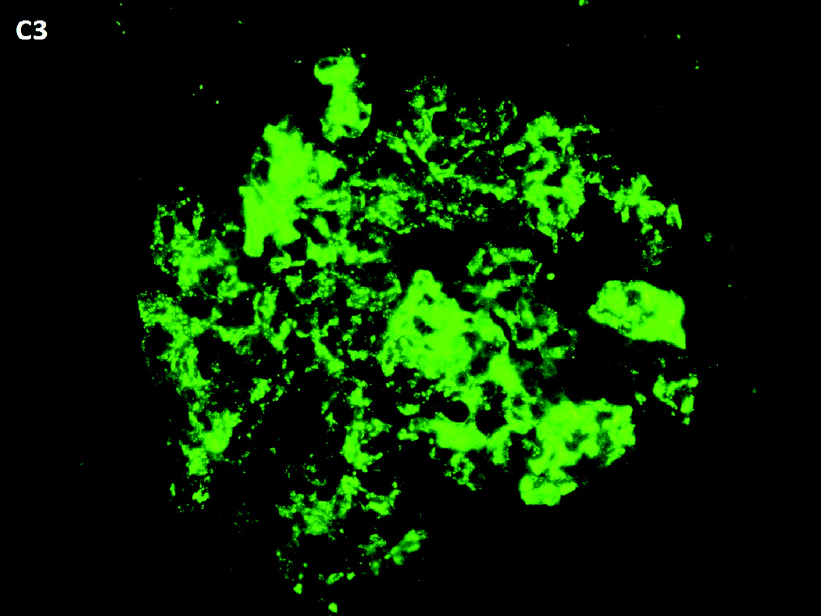 |
| Figure 2: Immunofluorescence showing mesangial and capillary C3 deposits (‘starry sky pattern’) |
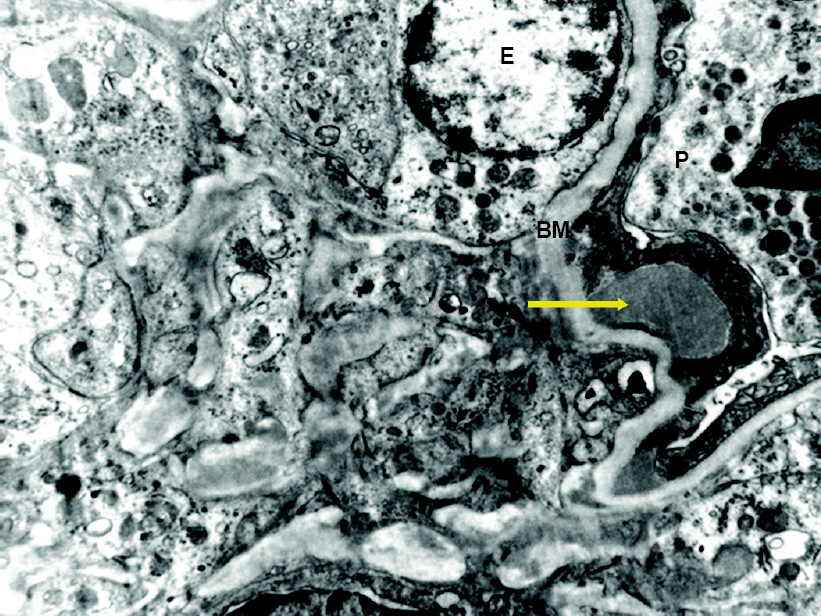 |
| Figure 3: Electron microscopy showing dome-shaped sub-epithelial hump E endothelial cell P podocyte BM glomerular basement membrane |
Results
Demographics
Sixty-one new patients with a diagnosis of IRGN were included in the study. Of these, 8 patients who did not meet the mandatory 3-month follow-up period and another 2 patients who died within 3 months (one due to catheter-related sepsis and another due to massive gastrointestinal bleed) were excluded from the analysis. Six patients with IgA-dominant IRGN were also excluded. Forty-five patients of biopsy-proven adult IRGN were included for analysis, of whom 25 patients satisfied three criteria and 20 patients satisfied four criteria. Their mean (SD) age was 41.5 (15.22) years (range 18–70 years) and 25 were females.
Clinical features
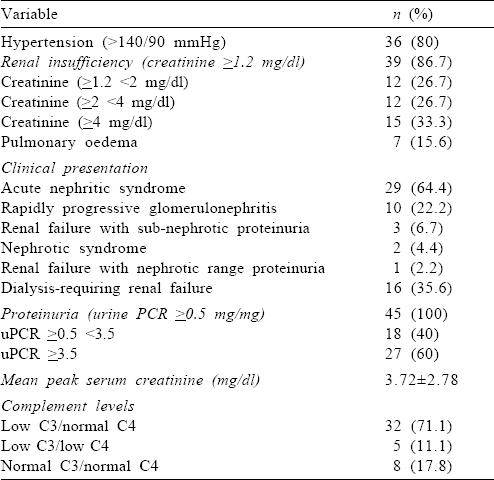
At presentation, all the patients had oedema and 38 (84.4%) patients had oliguria. Of the 37 patients (80%) with hypertension, 27 patients had new-onset hypertension and 9 patients had pre-existing hypertension. Thirty-five (77.8%) patients had haematuria at presentation (microhaematuria 21, macro-haematuria 14). Leucocyturia was present in 27 (60%) patients, and leucocyturia without haematuria was present in 7 (15.6%) patients. Thirty-nine (86.7%) patients had renal insufficiency (serum creatinine ≥1 mg/dl) at presentation, and acute nephritic syndrome was the most common presentation. The mean (SD) serum albumin was 3.25 (0.56) g/dl. Hypoalbuminaemia (<3 g/dl) was present in 15 patients. The clinical and laboratory parameters are given in [Table - 1].
The predisposing risk factors for infection were diabetes mellitus (7 patients), alcohol intake (7 patients) and severe anaemia (3 patients). None of the patients had other known predisposing factors such as malignancy, cirrhosis, HIV infection, intravenous drug abuse or immunosuppressive intake.
In patients with documented infection preceding renal disease [Table - 2], the mean (SD) time from infection to onset of renal disease was 16.5 (8.9) days (range 10–30). [Table - 2] summarizes the probable source of infection in IRGN patients.
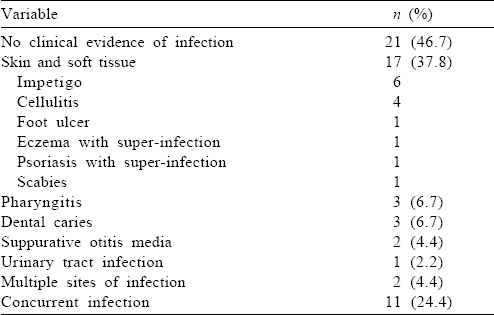
In the 11 patients with documented concurrent infection, the organisms isolated were Streptococcus species (3), Staphylococcus aureus (2), Klebsiella pneumoniae (1), Pseudomonas (1) and Acinetobacter (1). No organism could be isolated in the remaining 3 patients.
Histopathological features
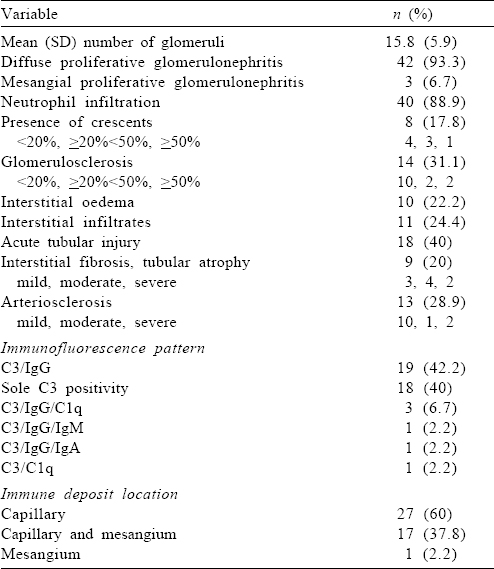
Light microscopic and IF characteristics are given in [Table - 3]. The most common pattern observed was diffuse endocapillary proliferation. Diabetic glomerulosclerosis was noted in 4 patients (RPS class II in 2 and RPS class III in 2), in addition to features of IRGN.
Treatment
All patients were treated with restricted salt and fluid intake, diuretics, antihypertensive drugs and renin angiotensin system blockers. Patients with an active, concurrent infectious source were treated with antibiotics. Three patients underwent extraction of dental caries, and 1 patient underwent modified radical mastoidectomy for chronic suppurative otitis media. Only 11 patients received steroids in addition to the other above-mentioned measures. Among dialysis-requiring patients, a mean (SD) of 5.47 (2.7) intermittent haemodialysis sessions (range 2–9) were required. The mean (SD) duration of hospital stay was 10.5 (7.9) days.
Outcome
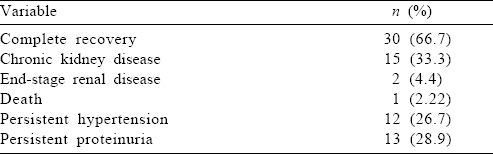
The mean (SD) follow-up period was 45.7 (20) weeks (range 16–89). Thirty-nine patients (86.7%) had a more than 24-week follow-up. None of the patients had persistence of haematuria. Persistent proteinuria with normal renal function was present in 2 patients (4.4%).
Among the 16 patients who required dialysis at presentation, 8 (50%) developed CKD, with one of them reaching ESRD. Of the 39 patients who had renal failure at presentation, 24 (61.5%) had complete recovery. Of the 15 patients with CKD, 11 patients were in stage 3, 1 was in stage 4, and 3 were in stage 5 at last follow-up.
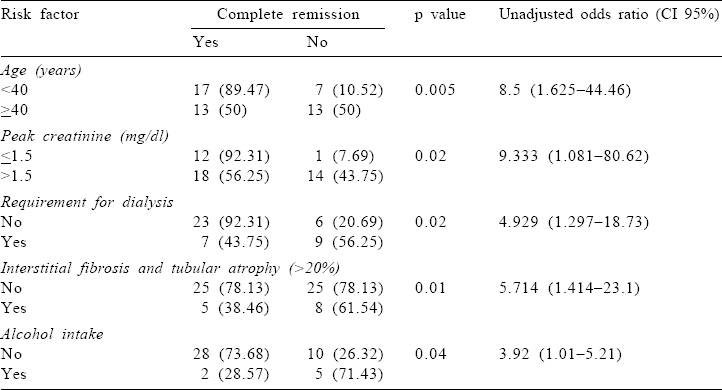
Age >40 years, alcohol intake, peak creatinine >1.5 mg/dl, a requirement for dialysis at presentation and presence of moderate-to-severe interstitial fibrosis with tubular atrophy (IFTA) were associated with failure to attain complete recovery (univariate analysis by the chi-square test). Subjecting these variables to logistic regression analysis, a requirement for dialysis at presentation (p=0.047) and presence of moderate-to-severe IFTA (p=0.037) were identified as independent risk factors.
Discussion
Our study has highlighted the changing epidemiological and clinicopathological presentation of IRGN. The female predominance (1:1.25) observed in our study cohort is contrary to the male predominance observed in other reported series,[2],[8],[9],[10],[15] except one by Srisawat et al. from Thailand.[18] Diabetes mellitus and alcohol intake are major predisposing risk factors for IRGN in adults, whereas other infrequent risk factors are malignancy, severe malnutrition, prosthetic heart valve, intravenous drug use and AIDS.[2],[9],[10] In our patients, diabetes, alcohol intake and severe anaemia were present in 15.6%, 15.6%, and 6.7% of patients, respectively. None of the other predisposing factors were present in them.
Adult IRGN patients can have various sources of infection including upper respiratory tract, skin, lung, heart, urinary tract, oral cavity, bone and deep-seated visceral or somatic abscess.[17] Among them, upper respiratory tract and skin are the most common sites. In our study, the most common sites of infection were skin (37.8%) followed by upper respiratory tract (6.7%) and teeth (6.7%). Skin infection predominates in the elderly and people with diabetes,[9] whereas dental infection predominates in those consuming alcohol.[2],[15] One patient had scabies without evidence of super-added bacterial infection, which is rare in the reported literature.[19] Streptococcus and Staphylococcus were the most common causative organisms in our study, which is in concordance with most other series.[2],[9],[10] Despite the extensive systematic screening for an infective focus, 46.7% of our patients did not have any clinical evidence of infection. This concurs with our previously reported retrospective study of adult IRGN.[16] However, this is in contrast to most other reported series.[2],[8],[9],[10],[15]
Our series had peripheral oedema in 100%, hypertension in 80% and microhaematuria in 46.7% of patients, which is in agreement with the findings from other reported case series.[8],[9],[10] Nephrotic range proteinuria was present in 60% of patients in our study, which is high compared to other case series. At presentation, 86.7% had renal insufficiency and 35.6% had need for dialysis in our study. Renal insufficiency and a requirement for dialysis at presentation are common in adults, particularly in the elderly.[9]
Hypocomplementaemia is known to occur in 35%–80% of adults with IRGN,[2],[8],[9],[10] and serum complement levels usually normalize within 2 months of presentation. In our study, hypo-complementaemia (low C3/C4) was observed in 82.2% of patients, and the levels normalized in all of them. None of our patients were positive for ANA, ANCA or cryoglobulins.
The most common light microscopy pattern in our study was diffuse endocapillary proliferative glomerulonephritis. In IF, sole C3 positivity was present in 40%.
IgA-dominant IRGN is a separate subclass of IRGN commonly occurring in the elderly and people with diabetes. It portends an invariably poor prognosis as evidenced by various studies including one case series from our institute.[20] We have excluded this entity from this study.
In our study, 33.3% of adult IRGN progressed to CKD. In various reported case series from across the globe, IRGN progresses to CKD in 8%–54% of cases.[2],[8],[9],[10],[15],[21]
Previous studies have enlisted various poor prognostic factors in IRGN that can predict the progression to CKD. These risk factors include diabetes mellitus, underlying diabetic glomerulosclerosis, a requirement for dialysis at presentation, presence of IFTA, hypoalbuminaemia, higher serum creatinine at biopsy, presence of underlying comorbid conditions, older age and crescents in the biopsy.[8],[9],[16] In our study, age >40 years, peak serum creatinine >1.5 mg/dl, a requirement for dialysis at presentation, IFTA>20% and alcohol intake were identified as risk factors by univariate analysis. By logistic regression analysis, a requirement for dialysis at presentation and IFTA >20% were risk factors.
No single clinical or pathological finding is pathognomonic for IRGN, and the present consensus is to use the clinico-pathological diagnostic criteria proposed by Nasr.[17] The strengths of our study are application of stringent diagnostic criteria, serial C3 levels and the prospective design. All except one[19] previous studies addressing the issue of outcome of IRGN have been retrospective. They have included presumed cases of IRGN even without biopsy, which could have included few misdiagnosed cases. C3 glomerulonephritis has to be differentiated from IRGN, especially in the situation of a worse prognosis of a previously benign disease.
Another strength of our study is that a structured extensive screening for any occult infectious focus was done in all patients, and occult sources of infection such as suppurative otitis media and dental caries were identified in 7 (15.6%) patients.
Conclusion
IRGN is no longer a benign disease in adults. Patients with IRGN will benefit from an extensive search for any occult infectious focus. Given the increasing incidence and relatively poor prognosis, more research is required on this important clinical entity.
Conflicts of interest. None declared
| 1. | Nadasdy T, Hebert LA. Infection-related glomerulonephritis: Understanding mechanisms. Semin Nephrol 2011;31:369–75. [Google Scholar] |
| 2. | Montseny JJ, Meyrier A, Kleinknecht D, Callard P. The current spectrum of infectious glomerulonephritis. Experience with 76 patients and review of the literature. Medicine (Baltimore) 1995;74:63–73. [Google Scholar] |
| 3. | Hinglais N, Garcia-Torres R, Kleinknecht D. Long-term prognosis in acute glomerulonephritis. The predictive value of early clinical and pathological features observed in 65 patients. Am J Med 1974;56:52–60. [Google Scholar] |
| 4. | Lien JW, Mathew TH, Meadows R. Acute post-streptococcal glomerulonephritis in adults: A long-term study. Q J Med 1979;48:99–111. [Google Scholar] |
| 5. | Vogl W, Renke M, Mayer-Eichberger D, Schmitt H, Bohle A. Long-term prognosis for endocapillary glomerulonephritis of poststreptococcal type in children and adults. Nephron 1986;44:58–65. [Google Scholar] |
| 6. | Chugh KS, Malhotra HS, Sakhuja V, Bhusnurmath S, Singhal PC, Unni VN, et al. Progression to end stage renal disease in post-streptococcal glomerulonephritis (PSGN) – Chandigarh study. Int J Artif Organs 1987;10:189–94. [Google Scholar] |
| 7. | Glassock RJ, Alvarado A, Prosek J, Hebert C, Parikh S, Satoskar A, et al. Staphylococcus-related glomerulonephritis and poststreptococcal glomerulo-nephritis: Why defining “post” is important in understanding and treating infection-related glomerulonephritis. Am J Kidney Dis 2015;65:826–32. [Google Scholar] |
| 8. | Moroni G, Pozzi C, Quaglini S, Segagni S, Banfi G, Baroli A, et al. Long-term prognosis of diffuse proliferative glomerulonephritis associated with infection in adults. Nephrol Dial Transplant 2002;17:1204–11. [Google Scholar] |
| 9. | Nasr SH, Fidler ME, Valeri AM, Cornell LD, Sethi S, Zoller A, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol 2011;22:187–95. [Google Scholar] |
| 10. | Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D’Agati VD, et al. Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine (Baltimore) 2008;87:21–32. [Google Scholar] |
| 11. | Whitworth JA, Morel-Maroger L, Mignon F, Richet G. The significance of extracapillary proliferation. Clinicopathological review of 60 patients. Nephron 1976;16:1–9. [Google Scholar] |
| 12. | Beaufils M, Morel-Maroger L, Sraer JD, Kanfer A, Kourilsky O, Richet G, et al. Acute renal failure of glomerular origin during visceral abscesses. N Engl J Med 1976;295:185–9. [Google Scholar] |
| 13. | Beaufils M. Glomerular disease complicating abdominal sepsis. Kidney Int 1981;19:609–18. [Google Scholar] |
| 14. | Peel R, Sellars L, Long ED, Bhandari S. A man with backache and renal failure. Am J Kidney Dis 2003;41:E1. [Google Scholar] |
| 15. | Keller CK, Andrassy K, Waldherr R, Ritz E. Postinfectious glomerulonephritis – Is there a link to alcoholism? Q J Med 1994;87:97–102. [Google Scholar] |
| 16. | Natarajan G, Ramanathan S, Jeyachandran D, Balasubramaniyan T, Srinivasa Prasad ND, Thanigachalam D, et al. Follow-up study of post-infectious glomerulo-nephritis in adults: Analysis of predictors of poor renal outcome. Saudi J Kidney Dis Transpl 2014;25:1210–16. [Google Scholar] |
| 17. | Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulo-nephritis in adults. Kidney Int 2013;83:792–803. [Google Scholar] |
| 18. | Srisawat N, Aroonpoonsub L, Lewsuwan S, Kanjanabuch T, Avihingsanon Y, Praditpornsilpa K, et al. The clinicopathology and outcome of post-infectious glomerulonephritis: Experience in 36 adults. J Med Assoc Thai 2006;89 (2 Suppl):S157–62. [Google Scholar] |
| 19. | Trivedi M, Pasari A, Chowdhury AR, Kurien AA, Pandey R. The epidemiology, clinical features, and outcome of infection-related glomerulonephritis from East India: A single center experience. Indian J Nephrol 2017;27:307–12. [Google Scholar] |
| 20. | Dhanapriya J, Balasubramaniyan T, Maharajan SP, Dineshkumar T, Sakthirajan R, Gopalakrishnan N, et al. IgA-dominant infection-related glomerulonephritis in India: A single-center experience. Indian J Nephrol 2017;27:435–9. [Google Scholar] |
| 21. | Okpechi I, Swanepoel C, Duffield M, Mahala B, Wearne N, Alagbe S, et al. Patterns of renal disease in Cape Town South Africa: A 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant 2011;26:1853–61. [Google Scholar] |
Fulltext Views
2,395
PDF downloads
477




