Translate this page into:
Coexistence of BCR–ABL1 reassortment and JAK2 V617F mutation in chronic myeloid leukaemia
Correspondence to JIANJUN YANG CHEELOO; yangjianjunsd@163.com
[To cite: Cheeloo JY, Bi K, Zhang Z. Coexistence of BCR–ABL1 reassortment and JAK2 V617F mutation in chronic myeloid leukaemia. Natl Med J India 2024;37:325–6. DOI: 10.25259/NMJI_61_2022]
Abstract
Myeloproliferative neoplasms (MPNs) can be classified into two major categories, namely chronic myeloid leukaemia (CML) and Philadelphia-negative MPNs (PN-MPNs). BCR– ABL1 reassortment is an irreplaceable indicator for the diagnosis of typical CML, while the V617F mutation on the Janus kinase 2 (JAK2) gene is common in PN-MPNs patients. Generally, these two genetic abnormalities are considered unable to coexist. We report a patient with CML who had both genetic changes, suggesting that when tyrosine kinase inhibitors (TKIs) monotherapy cannot obtain satisfactory treatment outcomes in CML patients, another possibility besides disease progression is a mutation on the JAK2 V617F gene.
INTRODUCTION
Few patients have been reported to contain both BCR–ABL1 reassortment and JAK2 V617F mutation in the development of myeloproliferative neoplasms (MPNs). The correlation between these two genetic abnormalities should be studied even if the chance of their coexistence is rare since different treatment strategies may apply should such coexistence happen.1
THE CASE
A 50-year-old woman was diagnosed with CML (chronic stage, Sokal 0.99, medium danger) four years ago. At the time of diagnosis, chromosome analysis showed a karyotype of 46,XX,t(9:22)(q34;q11.2), and the BCR–ABL1/ABL1 ratio was 16% with negative results for JAK2 V617F mutation. She was given imatinib 0.4 g orally once a day for 3 months before reexamination. The results now showed that the BCR–ABL1 indicator was negative. Following this, the patient continued to take imatinib orally at a dose of 0.4 g once a day. Six months ago, she was admitted to our hospital again. Examination found spleen enlargement below the ribs (6 cm). Routine blood tests revealed a white blood cell (WBC) count of 17.72×109 cells/L, a neutrophil count of 14.43×109 cells/L, a haemoglobin (Hb) concentration of 15.2 g/dl, and a platelet (PLT) count of 504×109/L. Polymerase chain reaction (PCR) was negative for BCR– ABL1 fusion gene and there were no abnormalities in the ABL kinase domain, and the chromosome karyotype was 46,XX.
The patient was considered CML (acceleration phase) in the beginning, so imatinib was replaced by 100 mg dasatinib taken orally once a day. However, the patient developed diarrhoea, so the dose was reduced to 50 mg once a day. Nevertheless, the disease continued to progress and the patient also had chest tightness. Examination revealed low breathing sounds in the right lung and spleen enlargement to the navel. Computed tomography (CT) scans on the chest revealed marked thoracic effusion in the right chest. Routine blood tests showed a WBC count of 19.43×109 cells/L, a Hb of 16.1 g/dl, and a PLT count of 535×109/L. Bone marrow biopsy found active bone marrow hyperplasia and advantaged myeloid proliferation (Fig. 1A). Specifically, marked hyperplasia was not observed for naive cells but for fibrous tissues, and the Gomori staining result was positive (++–+++; Fig. 1B). Bone marrow flowcytometry revealed no abnormal immunophenotypes. The rate of JAK2 V617F mutations was 8% by PCR and Sanger DNA sequencing. Therefore, we considered that the progression of the patient’s symptoms was related to the JAK2 V617F mutation.
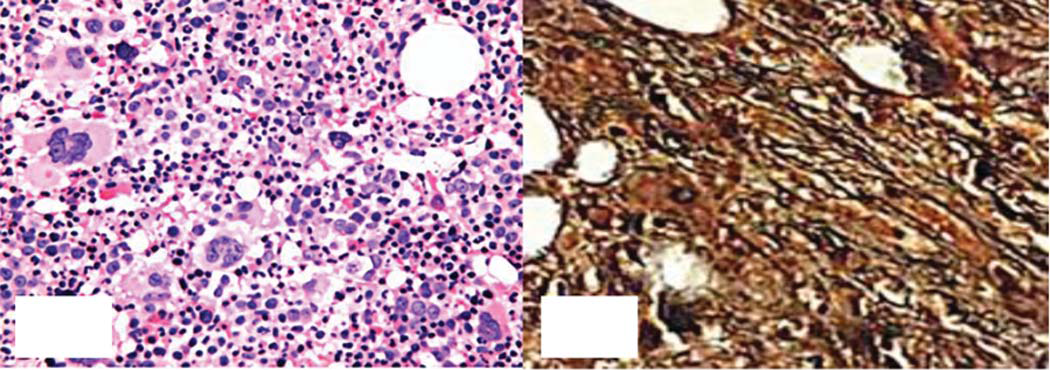
- Bone marrow biopsy. A: Haematoxylin and eosin staining shows active proliferation of granulocytes and megakaryocytes (400×); B: Gomori staining is positive (++–+++; 400×)
As the patient was confirmed CML in the remission state, the thoracic effusion was regarded as an outcome of dasatinib administration, so the patient received flumatinib, 0.6 g orally once a day. She also underwent drainage of the effusion and diuretics. The patient was also treated with 0.5 g hydroxyurea twice a day to reduce cell counts. With these symptomatic treatments, the patient’s symptoms alleviated and she was discharged after the peripheral blood cell counts were normal. Regular follow-up found normal peripheral blood cell counts, and the spleen returned to normal size in 2 months. Three months after discharge, BCR–ABL1 remained negative on PCR, but the percentage of JAK2 V617F mutation rose to 12%. At present, the patient is still taking flumatinib 0.6 g once a day and hydroxyurea 0.5 g twice a day and is stable. Despite that, the level of JAK2 V617F mutation continues to increase and bone marrow fibrosis persists. Therefore, the patient is likely to have a poor long-term outcome.
DISCUSSION
The mosaic cancer gene BCR–ABL1 has a high activity towards tyrosine kinase (Tyk), and TKIs can inhibit the kinase activity of BCR–ABL1, leading to a significant increase in the survival of patients with CML with continuous response.2 Point mutations in the BCR–ABL1 kinase domain are one of the most common reasons for drug resistance, and 4 mutation site types have been identified, namely the phosphate-binding loop (M244, G252, Y253, E255), the gatekeeper residue (imatinib binding sites T315 and F317), the SH2 binding region and C-lobe (mutations near the catalytic domain: M351 and F359), and the activation loop (H396).3 The efficacy of imatinib treatment is quite impaired in patients with these mutations, and multiple second-generation TKIs have been developed to overcome the lack of effect of imatinib.
JAK2 V617F mutation could lead to unsatisfactory treatment outcomes in patients with CML on treatment with TKIs.4 No optimal treatment strategy is available for JAK2 V617F-positive CML patients, and TKIs remain the preferred treatment for them.5 Ali et al.6 reported a patient diagnosed with both JAK2-positive and BCR–ABL-positive CML that had a good haematological and cytogenetic response to dasatinib. Whether these patients will benefit from ruxolitinib, a JAK2 inhibitor, remains questionable.
In our patient, the BCR–ABL fusion gene was found at the time of diagnosis of CML. JAK2 V617F emerged during imatinib treatment, and a remarkable PLT increase was observed. Therefore, we hypothesize that BCR–ABL1 reassortment and JAK2 V617F mutation may co-exist in different clones grown independently. Bee et al.7 suggested that the JAK2 V617F mutation would disappear when BCR–ABL1 mRNA transcription was at the maximum. Once the content of BCR– ABL1 mRNA decreased as a result of imatinib treatment, the JAK2 V617F mutation was observed again, which is consistent with the findings in our patient.
Conclusion
When CML patients do not show major improvement after treatment with TKIs or have a repetitive increase in WBC and/or PLT as well as splenic enlargement after an initial response, the possibility of secondary disease or transformation to other MPN types rather than disease progression or drug resistance must be excluded to ensure the best treatment outcome for these patients. Besides, when diagnosing and treating CML, special attention should be paid to identifying patients who are positive for JAK2 mutations and adjusting the treatment strategies for them accordingly.
Conflicts of interest
None declared
Consent
We obtained consent from the patient to publish this report.
References
- The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018;8:15.
- [CrossRef] [PubMed] [Google Scholar]
- The evolving landscape of frontline therapy in chronic phase chronic myeloid leukemia (CML) Curr Hematol Malig Rep. 2021;16:448-54.
- [CrossRef] [PubMed] [Google Scholar]
- Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938-42.
- [CrossRef] [PubMed] [Google Scholar]
- Coexisting driver mutations in MPN: Clinical and molecular characteristics of a series of 11 patients. Hematology. 2018;10:785-92.
- [CrossRef] [PubMed] [Google Scholar]
- Colony-forming cell assay detecting the co-expression of JAK2V617F and BCR-ABL1 in the same clone: A case report. Acta Haematol. 2019;141:261-7.
- [CrossRef] [PubMed] [Google Scholar]
- A case report of BCR-ABL-JAK2-positive chronic myeloid leukemia with complete hematological and major molecular response to dasatinib. Case Rep Oncol. 2021;14:690-4.
- [CrossRef] [PubMed] [Google Scholar]
- A man with concomitant polycythaemia vera and chronic myeloid leukemia: The dynamics of the two disorders. Int J Hematol. 2010;91:136-9.
- [CrossRef] [PubMed] [Google Scholar]




