Translate this page into:
Consolidation chemotherapy after concurrent chemoradiotherapy in locally advanced nonsquamous non-small cell lung cancer: When, in whom and how much?
Corresponding Author:
Bivas Biswas
Department of Medical Oncology, Tata Medical Center, Kolkata, West Bengal
India
bivasbiswas@gmail.com
| How to cite this article: Biswas B, Dabkara D, Ganguly S, Prasad E. Consolidation chemotherapy after concurrent chemoradiotherapy in locally advanced nonsquamous non-small cell lung cancer: When, in whom and how much?. Natl Med J India 2017;30:26-27 |
Senan S, Brade A, Wang L-h, Vansteenkiste J, Dakhil S, Biesma B, Aguillo MM, Aerts J, Govindan R, Rubio-Viqueira B, Lewanski C, Gandara D, Choy H, Mok T, Hossain A, Iscoe N, Treat J, Koustenis A, Antonio BS, Chouaki N, Vokes E. (VU Medical Center, Amsterdam; Jeroen Bosch Hospital, s-Hertogenbosch; and Erasmus MC Rotterdam/Amphia Hospital Breda, Breda, the Netherlands; Princess Margaret Hospital, University of Toronto; Eli Lilly Canada, Toronto, Ontario, Canada; Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing; The Chinese University of Hong Kong, Shatin, Hong Kong Special Administrative Region, People's Republic of China; University Hospital KU Leuven, Leuven, Belgium; Cancer Center of Kansas, Wichita, Kansas; Washington University School of Medicine, Saint Louis, Missouri; University of California, Davis Health System, Sacramento, California; UT Southwestern Medical Center, Dallas, Texas; Eli Lilly and Company, Indianapolis, Indiana; University of Chicago, Chicago, Illinois, USA; Hospital of Navarre, Irunlarrea, Pamplona; Hospital Universitario Quiron Madrid; Eli Lilly and Company, Madrid, Spain; Charing Cross Hospital, London, United Kingdom; Eli Lilly and Company, Neuilly-sur-Seine, France.) PROCLAIM: Randomized phase III trial of pemetrexed–cisplatin or etoposide–cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced non-squamous non-small-cell lung cancer. J Clin Oncol 2016;34:953–62.
Summary
Lung cancer is a leading cause of cancer-related mortality among men in India. Many patients present with an advanced stage of the disease. Non-small cell lung cancer (NSCLC) accounts for more than two-thirds of the instances of lung cancer with adenocarcinoma being the predominant histology. The standard treatment for patients with unresectable stage IIIA and IIIB NSCLC (approximately 30% of all cases) is concurrent chemotherapy with thoracic radiation (CCRT). Platinum doublet is the backbone of concurrent chemotherapy regimens used with thoracic radiation.[1] For non-squamous histology of NSCLC, other regimens include carboplatin–pemetrexed and cisplatin–pemetrexed.[2] No guidelines exist on whether or not consolidation chemotherapy is beneficial after CCRT in this subgroup of patients.
This study (PROCLAIM) was a randomized phase 3 trial in histologically/cytologically confirmed stage IIIA/B non-squamous NSCLC, aged ≥18 years and Eastern Cooperative Oncology Group performance status of 0 and 1. Patients were randomized to two arms to receive CCRT followed by consolidation chemotherapy. Arm A received pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 intravenously (i.v.) every 3 weeks for three cycles with concurrent thoracic radiotherapy (60–66Gy) followed by pemetrexed consolidation every 3 weeks for 4 cycles. Arm B included standard therapy with etoposide 50 mg/m2 and cisplatin 50 mg/m2 i.v. every 4 weeks for two cycles with concurrent thoracic radiotherapy (60–66 Gy ) followed by two cycles of consolidation platinum-based doublet chemotherapy. The consolidation chemotherapy in arm B included: (i) etoposide–cisplatin (same dose and schedule as during concurrent treatment); (ii) vinorelbine–cisplatin (vinorelbine 30 mg/m2 i.v. on days 1 and 8 every 3 weeks and cisplatin i.v. on day 1 every 3 weeks); or (iii) paclitaxel–carboplatin (paclitaxel 200 mg/m2 i.v. every 3 weeks followed by carboplatin i.v. [area under the concentration–time curve, 6] every 3 weeks). Concurrent thoracic radiotherapy, 2 Gy/ fraction daily/5 days per week to a target dose of 60–66 Gy in 30 to 33 fractions, was started on day 1 of chemotherapy. Grade 3 radiation pneumonitis and radiotherapy interruptions of >7 days because of intercurrent illness required discontinuation of treatment. This was a superiority trial of arm A over arm B with overall survival (OS) as the primary end-point with 80% power to detect an OS hazard ratio (HR) of 0.74 with a type 1 error of 0.05. The study was terminated after enrolment of 598 patients (arm A 301, arm B 297) before planned accrual of 600 patients. This was because the trial was considered futile after an interim assessment that showed 173 deaths in 552 randomly assigned patients.
OS and progression-free survival (PFS) were analysed on an intent-to-treat basis. After a median follow-up of 22.2 months (arm A) and 22.6 months (arm B) at the time of data censoring, arm A was not superior to arm B in terms of OS (HR 0.98; 95% CI 0.79–1.20; median 26.8 v. 25.0 months; p=0.83) and PFS (HR 0.86; 95% CI 0.71–1.04; median 11.4 v. 9.8 months; p=0.13). The objective response rate was also not statistically different between the two arms (35.9% in arm A v. 33.0% in arm B). Arm A had a significantly lower incidence of any drug-related grade 3 to 4 adverse events (64.0% v. 76.8%; p=0.001).
Comment
We wish to highlight aspects that suggest that the results of this study may be biased. The consolidation chemotherapy regimens in the control arm were heterogeneous though similar chemotherapy was used during CCRT and consolidation in the experimental arm. The duration of consolidation therapy was also different in the two arms (12 weeks in arm A and 6 weeks in arm B) and also a single agent (pemetrexed) was used as consolidation in arm A compared with a platinum doublet in arm B. A recent randomized phase 3 study[3] and pooled analysis[4] concluded that consolidation chemotherapy after CCRT did not have any survival advantage with the use of different agents, such as docetaxel and/or cisplatin, gefitinib. However, consolidation chemotherapy has been traditionally added after CCRT with radiosensitizing agents due to their lack of systemic anticancer drug exposure[5],[6] and have shown some advantage in OS. Pemetrexed also showed a radiosensitizing property in vitro and its role as consolidation chemotherapy has never been tested in any properly designed prospective randomized studies in locally advanced NSCLC.[7],[8] The present study showed improved OS of >5 months compared with historical controls[1] but was similar to a previous phase 2 study of CCRT[8] with pemetrexed–cisplatin and failed to detect any difference with the control arm.
The median follow-up was short (22.2 months for pemetrexed–cisplatin v. 22.6 months for etoposide–cisplatin) to detect any true difference in OS, if any. The other possible reasons for not detecting any difference in OS may be due to a higher percentage of patients with adenocarcinoma being present than in previous studies[3],[7],[8],[9] (Niho et al.[1] with only 18 patients and Hanna et al.[10] did not report the percentage) and may suggest that consolidation chemotherapy works better in adenocarcinoma histology [Table - 1]. Also, the chemotherapy combinations and trial designs vary from one study to another with differences in end-points.
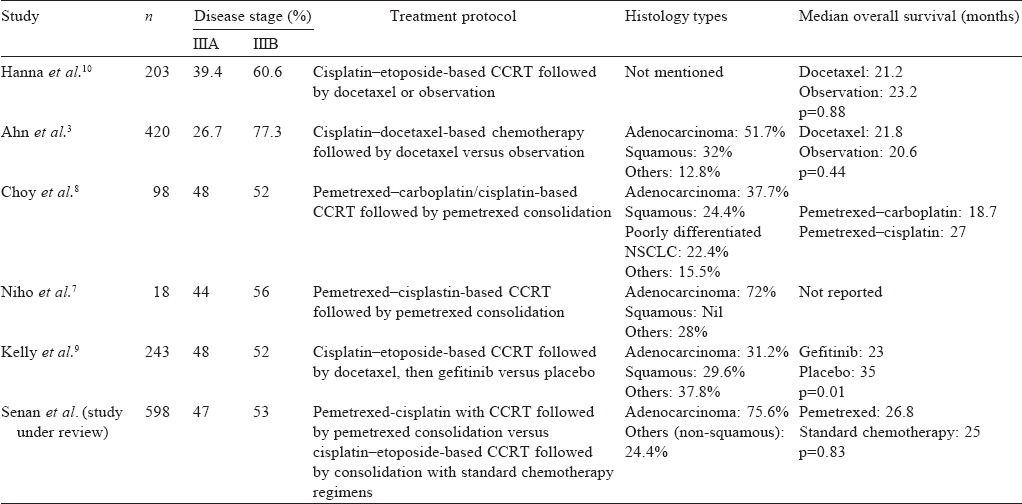
It would be prudent to know the time to distant failure in both the arms, as the principle of consolidation chemotherapy was to reduce systemic metastasis and thus PFS would have been the preferred primary end-point with this short follow-up instead of OS. The overall toxicity was also better in the pemetrexed–cisplatin arm and slightly more number of patients received consolidation therapy in this arm as compared to etoposide–cisplatin-based CCRT arm. Though it was not an objective, this study has still not answered the much awaited and debated question—’Is consolidation chemotherapy needed after CCRT?’, especially in those who received radiosensitizing agents such as pemetrexed. A prospective randomized trial in stage III unresectable non-squamous NSCLC with pemetrexed–cisplatin-based CCRT with or without pemetrexed-based consolidation therapy would be a realistic future study. It would provide a less toxic and effective CCRT regimen and can answer whether consolidation chemotherapy is beneficial in this particular subset of patients.
| 1. | Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–60. Erratum in: J Natl Cancer Inst 2012;104:79. [Google Scholar] |
| 2. | Govindan R, Bogart J, Stinchcombe T, Wang X, Hodgson L, Kratzke R, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011 ;29:3120–5. [Google Scholar] |
| 3. | Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol 2015;33:–6. [Google Scholar] |
| 4. | Tsujino K, Kurata T, Yamamoto S, Kawaguchi T, Kubo A, Isa S, et al. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol 2013;8:1181–9. [Google Scholar] |
| 5. | Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005;23:5883–91. Erratum in: J Clin Oncol 2006;24:1966. [Google Scholar] |
| 6. | Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJT0G0105. J Clin Oncol 2010;28:3739–45. [Google Scholar] |
| 7. | Niho S, Kubota K, Nihei K, Sekine I, Sumi M, Sekiguchi R, et al. Dose-escalation study of thoracic radiotherapy in combination with pemetrexed plus cisplatin followed by pemetrexed consolidation therapy in Japanese patients with locally advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2013;14:62–9. [Google Scholar] |
| 8. | Choy H, Schwartzberg LS, Dakhil SR, Garon EB, Gerber DE, Choksi JK, et al. Phase 2 study of pemetrexed plus carboplatin, or pemetrexed plus cisplatin with concurrent radiation therapy followed by pemetrexed consolidation in patients with favorable-prognosis inoperable stage IIIA/B non-small-cell lung cancer. J Thorac Oncol 2013;8:1308–16. [Google Scholar] |
| 9. | Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450–6. [Google Scholar] |
| 10. | Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. ; Hoosier Oncology Group; US Oncology. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26:5755–60. [Google Scholar] |
Fulltext Views
1,727
PDF downloads
1,081




