Translate this page into:
Data sharing Statements for Clinical Trials: A requirement of the International Committee of Medical Journal Editors
2 Representative and Past President, World Association of Medical Editors,
3 Scientific Editor-in-Chief, Ugeskrift for Laeger (Danish Medical Journal),
4 Chief Editor, PLOS Medicine,
5 Editor-in-Chief, Annals of Internal Medicine,
6 Deputy Editor, The Lancet,
7 Editor-in-Chief, Journal of Korean Medical Science,
8 Editor-in-Chief, Ethiopian Journal of Health Sciences,
9 Editor, Bulletin of the World Health Organization, Coordinator, WHO Press,
10 Editor-in-Chief, The BMJ (British Medical Journal),
11 Editor-in-Chief, New Zealand Medical Journal,
12 Editor, Revsita Médicade Chile (Medical Journal of Chile),
13 Editor-in-Chief, New England Journal of Medicine,
14 Editor-in-Chief, JAMA (Journal of the American Medical Association) and the JAMA Network,
15 Chief Scientific Editor, Deutsches Ärzteblatt (German Medical Journal) and Deutsches Ärzteblatt International,
16 Representative and Associate Director for Library Operations, National Library of Medicine,
Corresponding Author:
| How to cite this article: Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, Hong ST, Haileamlak A, Gollogly L, Godlee F, Frizelle FA, Florenzano F, Drazen JM, Bauchner H, Baethge C, Backus J. Data sharing Statements for Clinical Trials: A requirement of the International Committee of Medical Journal Editors. Natl Med J India 2017;30:121-124 |
The International Committee of Medical Journal Editors (ICMJE) believes there is an ethical obligation to responsibly share data generated by interventional clinical trials because trial participants have put themselves at risk. In January 2016, we published a proposal aimed at helping to create an environment in which the sharing of de-identified individual participant data becomes the norm. In response to our request for feedback we received many comments from individuals and groups.[1] Some applauded the proposals while others expressed disappointment they did not more quickly create a commitment to data sharing. Many raised valid concerns regarding the feasibility of the proposed requirements, the necessary resources, the real or perceived risks to trial participants, and the need to protect the interests of patients and researchers.
It is encouraging that data sharing is already occurring in some settings. Over the past year, however, we have learned that the challenges are substantial and the requisite mechanisms are not in place to mandate universal data sharing at this time. Although many issues must be addressed for data sharing to become the norm, we remain committed to this goal.
Therefore, ICMJE will require the following as conditions of consideration for publication of a clinical trial report in our member journals:
- As of 1 July 2018, manuscripts submitted to ICMJE journals that report the results of clinical trials must contain a data sharing statement as described below.
- Clinical trials that begin enrolling participants on or after 1 January 2019 must include a data sharing plan in the trial's registration. The ICMJE's policy regarding trial registration is explained at www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. If the data sharing plan changes after registration, this should be reflected in the statement submitted and published with the manuscript, and updated in the registry record.
Data sharing statements must indicate the following: whether individual de-identified participant data (including data dictionaries) will be shared; what data in particular will be shared; whether additional, related documents will be available (e.g. study protocol, statistical analysis plan, etc.); when the data will become available and for how long; by what access criteria data will be shared (including with whom, for what types of analyses and by what mechanism). Illustrative examples of data sharing statements that would meet these requirements are in [Table - 1].
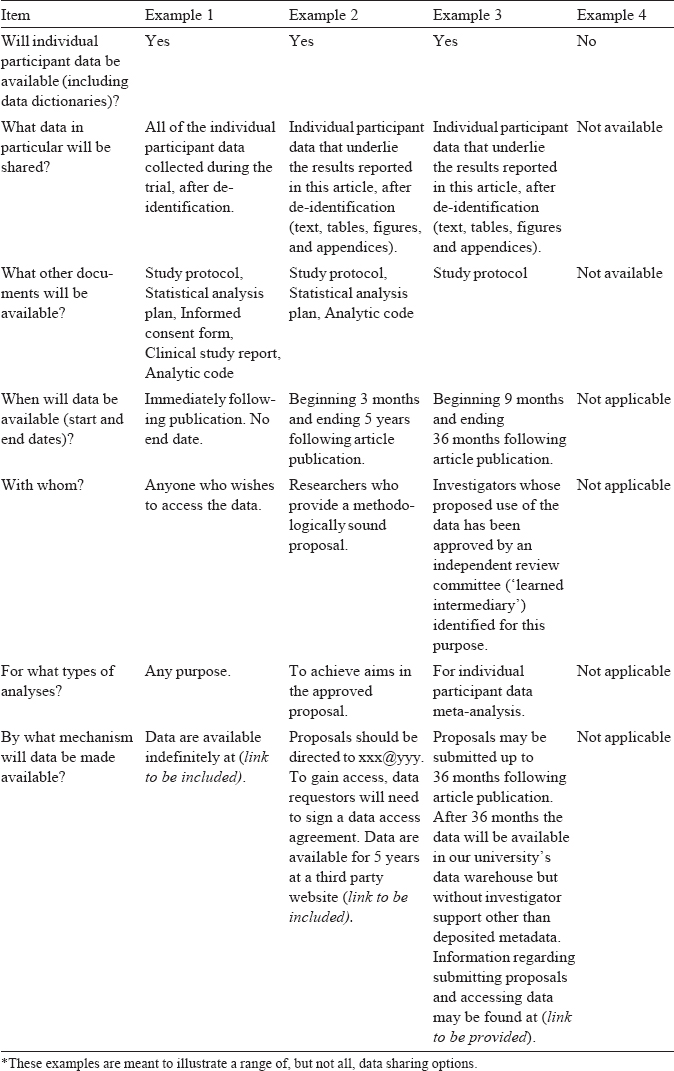
These initial requirements do not yet mandate data sharing, but investigators should be aware that editors may take into consideration data sharing statements when making editorial decisions. These minimum requirements are intended to move the research enterprise closer to fulfilling our ethical obligation to participants. Some ICMJE member journals already maintain, or may choose to adopt, more stringent requirements for data sharing.
Sharing clinical trial data is one step in the process articulated by WHO and other professional organizations as best practice for clinical trials: universal prospective registration; public disclosure of results from all clinical trials (including through journal publication); and data sharing. Although universal compliance with the requirement to prospectively register clinical trials has not yet been achieved and requires continued emphasis, we must work towards fulfilling the other steps of best practice as well—including data sharing.
As we move forward into this new norm where data are shared, greater understanding and collaboration among funders, ethics committees, journals, trialists, data analysts, participants, and others will be required. We are currently working with members of the research community to facilitate practical solutions to enable data sharing. The United States Office for Human Research Protections has indicated that provided the appropriate conditions are met by those receiving them, the sharing of de-identified individual participant data from clinical trials does not require separate consent from trial participants.[2] Specific elements to enable data sharing statements that meet these requirements have been adopted at ClinicalTrials.gov (https://prsinfo.clinicaltrials.gov/definitions.html# shareData). WHO also supports the addition of such elements at the primary registries of the International Clinical Trials Registry Platform. Unresolved issues remain, including appropriate scholarly credit to those who share data, and the resources needed for data access, the transparent processing of data requests, and data archiving. We welcome creative solutions to these problems at www.icmje.org.
We envision a global research community in which sharing de-identified data becomes the norm. Working towards this vision will help maximize the knowledge gained from the efforts and sacrifices of clinical trial participants.
Note: This article is being published simultaneously in Annals of Internal Medicine, BMJ (BritishMedical Journal), Bulletin of the World Health Organization, Deutsches Ärzteblatt (German Medical Journal), Ethiopian Journal of Health Sciences, JAMA (Journal of the American Medical Association), Journal of Korean Medical Science, New England Journal of Medicine, New Zealand Medical Journal, P LOS Medicine, The Lancet, Revista Médica de Chile (Medical Journal of Chile), and Ugeskriftfor Laeger (DanishMedical Journal).
Disclaimer: Dr Sahni's affiliation as representative and past president of the World Association of Medical Editors (WAME) does not imply endorsement by WAME member journals that are not part of the ICMJE.
In June 2017, this editorial from the International Committee of Medical Journal Editors (ICJME) was published simultaneously in member journals. Courtesy of the American College of Physicians, the publisher of Annals of Internal Medicine, The National Medical Journal of India has reprinted the version that appeared in Annals of Internal Medicine (Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, et al. Data sharing statements for clinical trials: A requirement of the International Committee of Medical Journal Editors. Ann Intern Med [Epub ahead of print 6 June 2017] doi: 10.7326/ M17-1028. http://annals.org/aim/article/2630766/Data sharing-statements-clinical-trials-requirement-international-committee-medical-journal (For more about the ICJME, see www.icjme.org).
| 1. | Taichman DB, Backus J, Baethge C, Bauchner H, de Leeuw PW, Drazen JM, et al. Sharing clinical trial data: A proposal from the International Committee of Medical Journal Editors [Editorial]. Ann Intern Med 2016;164:505-6. [PMID: 26792258] do1:10.7326/M15–2928. [Google Scholar] |
| 2. | Menikoff J. Letter from Jerry Menikoff, MD, JD, Director, Office for Human Research Protections, to ICMJE Secretariat. 7 March 2017. Available at www.icmje.org (accessed on 7 June 2017). [Google Scholar] |
Fulltext Views
1,525
PDF downloads
634




