Translate this page into:
Diabetic striatopathy in an adult with ketotic hyperglycaemia
Correspondence to SAUMYA GUPTA; g_saumya@yahoo.com
[To cite: Nahid E, Gupta S, Prasad K, Saha AK, Meher MP, Meena LP. Diabetic striatopathy in an adult with ketotic hyperglycaemia. Natl Med J India 2023;36:229–30. DOI: 10.25259/NMJI_282_20]
Abstract
Diabetic striatopathy (DS) is a rare and life-threatening mani- festation of diabetes. The disease commonly affects individuals of Asian descent, women and the elderly. DS is characterized by dyskinesias with basal ganglia hyperintensities on imaging. Despite being rare, prompt recognition of a hyperglycaemia- induced hemichorea–hemiballismus is essential because the symptoms are reversible with correction of hyperglycaemia. Diagnosis is based on blood analysis and neuroimaging findings. Laboratory tests reveal raised glycosylated haemoglobin (HbA1c) levels, which indicate poorly controlled diabetes. Neuroimaging provides suggestive findings of DS. It is usually associated with non-ketotic hyperglycaemia. We report a 50-year-old woman who presented with ketotic hyperglycaemia and left-sided hemichorea and partial seizures with secondary generalization.
INTRODUCTION
Acute severe hyperglycaemia causing neurological manifestation is well described, and the spectrum ranges from seizures to dyskinesias and coma. Diabetic striatopathy (DS) is a constellation of neurological abnormalities defined by the characteristic dyskinesia of chorea-ballism and striatal abnor- malities on neuroimaging.1 It has been described with non- ketotic hyperglycaemic hyperosmolar state, and instances in patients with ketotic hyperglycaemia are few. We report a patient with hemichorea–hemiballismus in a previously undiagnosed adult woman with ketotic hyperglycaemia.
THE CASE
A 50-year-old woman with no previous comorbid conditions presented to the emergency department with acute onset of involuntary movements of the left side of the body for the past 3 days. Evaluation revealed involuntary movements affecting both proximal and distal groups of muscles in the left upper and lower limbs. These were irregular, non-repetitive and not associated with loss of consciousness or disorientation, suggestive of left hemichorea–hemiballismus (video available at www.nmji.in). On the day of presentation, she developed left- sided deviation of the angle of the mouth followed by jerky movements of the left side of the body, which was followed by repetitive jerky tonic–clonic movements of the whole body, which was associated with up rolling of eyeballs and frothing from the mouth. This episode remained for 2 minutes, and then, the patient became drowsy. On examination, she was conscious with normal mental functions. Neurological examination revealed no obvious motor or sensory deficit. On admission, her blood glucose level was 620 mg/dl and her glycosylated haemoglobin (HbA1c) was 16.7%. Urine was positive for ketones. These laboratory results were consistent with ketotic hyperglycaemia. Arterial blood gas showed pH of 7.30 and bicarbonate of 16; high serum osmolarity (310 mOsm/kg) and contrast computed tomography (CT) head showed faint hyperdensity in the right caudate and lentiform nuclei (Fig. 1). On magnetic resonance imaging (MRI), the right caudate and lentiform nuclei were hyperintense on T1-weighed image (Fig. 2). Diffusion-weighted image (DWI) showed no diffusion abnormality in the cerebral parenchyma and the basal ganglia. DS was the final diagnosis based on the imaging findings and the laboratory results. After management with fluids and insulin infusion, the patient had an uneventful recovery within 24 hours. She was then shifted to a basal–bolus insulin regimen.

- Non-contrast computed tomography head: Circumscribed hyperdensities in the head of the right caudate and lentiform nuclei
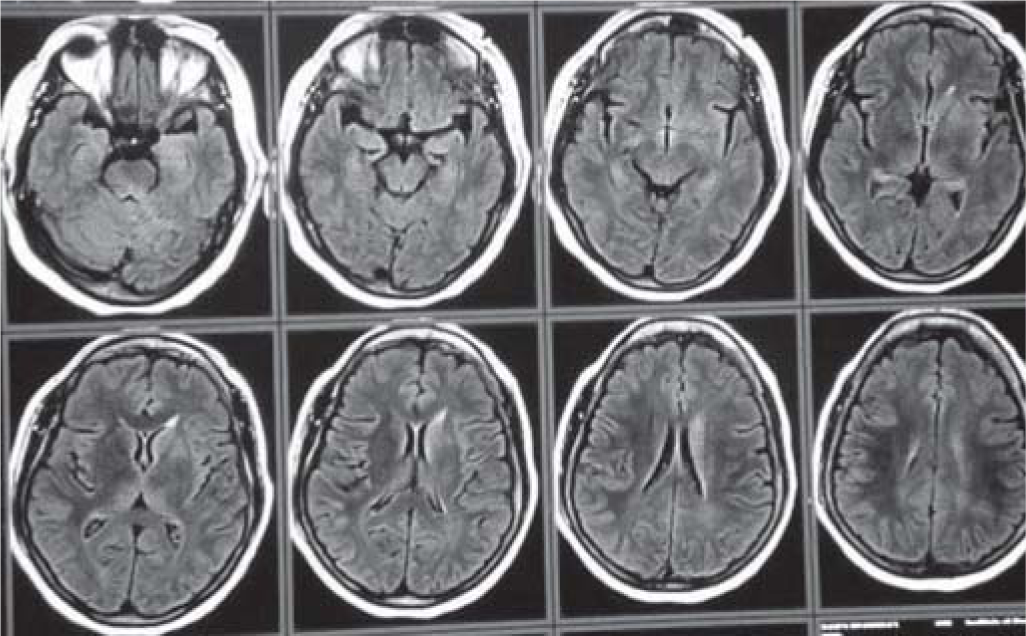
- Magnetic resonance imaging brain showing T1 hyperintensity in the right caudate and lentiform nuclei
DISCUSSION
Diabetic striatopathy denotes a condition in people with diabetes in whom there is a combination of striatal hyperintensity on T1-weighted MRI and contralateral movement disorder. It is an under-diagnosed complication of diabetes seen in patients with long-standing diabetes with poor control, more frequently encountered in non-ketotic hyperglycaemia and rarely in ketotic hyperglycaemia. This movement disorder can be one of the presenting features of diabetes.2–10 It is typically seen in elderly patients with type 2 diabetes.3 Women are affected more commonly than men.4 It is common in Asians, a feature that may suggest an underlying genetic predisposition.5 In India, where diabetes has a high prevalence, hyperglycaemia should be ruled out in all patients presenting with movement disorders.7
Patients usually present with acute onset of hemichorea–hemiballismus; however, in rare instances, a generalized movement disorder secondary to the involvement of bilateral basal ganglia or stroke-like complaints may be seen.5,6 The uniqueness of the presentation is that it is usually self-limiting with prompt management of the hyperglycaemia.
The pathogenesis of the abnormal movements is not fully understood. According to the suggested theory, hyperviscosity induced by hyperglycaemia causes a decrease in cerebral perfusion and an increase in anaerobic metabolism. In the hyperosmolar hyperglycaemic state, this shift causes the brain to metabolize gamma-aminobutyric acid (GABA) into succinic acid via the semialdehyde pathway, leading to rapid depletion of GABA levels,8 which is an inhibitory neurotransmitter. Thus, its depletion causes disinhibition of the thalamus by the medial globus pallidus, resulting in hyperkinetic movements. The striatopathy in diabetic ketoacidosis (DKA) is rare due to the presence of acetoacetate, which is able to produce GABA, thereby preventing its low levels.9 However, this theory is unable to explain unilateral basal ganglia involvement and the clinical presentation.
DS has characteristic neuroimaging findings predominantly seen in the putamen. The involvement of the caudate nucleus may be in association with the findings in the putamen.5 CT may show hyperattenuation in the basal ganglia. T1-weighted MRI typically shows increased signal intensity in the putamen/caudate nucleus.6 Diffusion-weighted images usually show no diffusion restriction.
Treatment usually involves correction of hyperglycaemia and maintaining adequate hydration.11 However, chorea can be controlled with glycaemic control alone in only one-fourth of patients. Others require additional anti-chorea drugs. These include neuroleptic agents (haloperidol/risperidone), dopamine- depleting agents (reserpine/tetrabenazine), GABAergic drugs (clonazepam/gabapentin) and selective serotonin reuptake inhibitors such as escitalopram.11 Haloperidol is the most common monotherapeutic agent used in DS-associated chorea. According to the literature, there is no consensus on how quick blood sugar control should be achieved in C-H-BG (Chorea, hyperglycaemia, basal ganglion) syndrome. A gradual improve- ment in blood glucose over a period of several days seems to be appropriate and leads to improvement of the choreiform movements while avoiding hypoglycaemia.
Conclusion
Movement disorders such as chorea, hemiballismus, hemichorea and choreoathetosis can occur in the settings of hyper- glycaemia. As hyperglycaemia is an easily treatable disorder, early recognition of hyperglycaemia-induced movement disorders is important and these have a good prognosis. Movement disorders can be one of the presenting features of diabetes mellitus. Hence, screening all patients who present with involuntary movements for hyperglycaemia, even in the absence of a history of diabetes, is important, especially in India, where diabetes is highly prevalent.
Conflicts of interest
None declared
References
- 'Diabetic striatopathy': Clinical presentations, controversy, pathogenesis, treatments, and outcomes. Sci Rep. 2020;10:1594.
- [CrossRef] [PubMed] [Google Scholar]
- Hemichorea-hemiballismus as the presenting manifestation of nonketotic hyperglycemia in an adolescent with undiagnosed type 2 diabetes mellitus. Indian J Endocrinol Metab. 2012;16(Suppl 1):S129-S131.
- [CrossRef] [PubMed] [Google Scholar]
- Chorea, hyperglycemia, basal ganglia syndrome (C-H-BG) in an uncontrolled diabetic patient with normal glucose levels on presentation. Am J Case Rep. 2014;15:143-6.
- [CrossRef] [PubMed] [Google Scholar]
- Chorea associated with nonketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: A meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200:57-62.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging in patients with abnormal blood glucose levels. AJNR Am J Neuroradiol. 2014;35:833-40.
- [CrossRef] [PubMed] [Google Scholar]
- Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17:1057-64.
- [Google Scholar]
- Movement disorders and diabetes, a study from south India. Internet J Neurol. 2012;14(1):1-5.
- [CrossRef] [Google Scholar]
- Hemiballism-hemichorea and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry. 1994;57:748-50.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical onset of diabetes in a teenage girl: A case report. Cases J. 2008;1:425.
- [CrossRef] [PubMed] [Google Scholar]
- Irreversible hemichorea-hemiballism in a case of nonketotic hyperglycemia presenting as the initial manifestation of diabetes mellitus. Tremor Other Hyperkinet Mov (NY). 2016;6:393.
- [CrossRef] [Google Scholar]
- 'Diabetic striatopathy' and ketoacidosis: Report of two cases and review of literature. Diabetes Res Clin Pract. 2017;128:1-5.
- [CrossRef] [PubMed] [Google Scholar]




