Translate this page into:
Effect of moxifloxacin on QTc interval in adults with pulmonary tuberculosis
2 Indian Council of Medical Research, New Delhi, India
Corresponding Author:
Dina Nair
Department of Clinical Research, National Institute for Research in Tuberculosis, Chennai, Tamil Nadu
India
dinanair73@yahoo.co.in
| How to cite this article: Nair D, Velayutham B, Marimuthu M, Navaneethapandian PG, Chinnaiyan P, Jawahar MS, Swaminathan S. Effect of moxifloxacin on QTc interval in adults with pulmonary tuberculosis. Natl Med J India 2018;31:58-59 |
The fluoroquinolone group of drugs is extensively used to treat acute bacterial infections such as sinusitis and acute exacerbations of lower respiratory tract infections. These drugs have the propensity to prolong the QTc interval in a dose-dependent manner by inhibition of potassium channels, especially the delayed rectifier potassium current (IKR), coded by the human ether-a-go-go gene.[1],[2] QT interval prolongation predisposes to ventricular tachyarrhythmia and Torsades de pointes (TdP). This assumes clinical importance in the presence of risk factors such as advanced age, being a woman, familial long QT syndromes, congestive heart failure, renal and liver dysfunction, electrolyte abnormalities and interactions with multiple drugs that prolong the QT interval.[3] Hence, QTc prolongation is monitored and used as an end-point in clinical trials.[4]
Moxifloxacin (MFX), a 8-methoxyquinolone with promising antimycobacterial activity, is being evaluated in many randomized clinical trials for shortening the duration of treatment for tuberculosis (TB).[5],[6] Even though there are data on the cardiac toxicity of short-duration MFX for acute bacterial infections, there is little information on the safety of MFX when given for a longer duration.
We did a trial to study the efficacy and safety of MFX-containing regimens (CTRI/2008/091/000024). We present an analysis on the serial electrocardiogram (ECG) data in the patients enrolled in this trial.
Methods
Newly diagnosed sputum-positive pulmonary TB, HIV-seronegative patients were randomized to four MFX regimens of 3- and 4-month duration or a 6-month standard regimen after obtaining written informed consent.[6] Patients treated in MFX regimens received a daily dose of 400 mg along with other first-line anti-TB drugs. Patients with a prior history of cardiac disease, an abnormal ECG or with a QTc of >450 ms at baseline were not enrolled.
ECG was recorded at baseline or pre-treatment, 1, 2, 3 and 4 months’ post-treatment by a central ECG provider (PageWriter 100) that gave an automated report. The timing and registration technique for ECGs were standardized for all patients. The ECGs were recorded for 10 seconds. Patients were recumbent for at least 5 minutes before each 12-lead ECG evaluation. All ECGs were reviewed by the treating doctor before providing ensuing treatment. Unscheduled ECG evaluations were made for participants having a QTc >450 ms during treatment follow-up, and if persistently high, they were referred for a cardiologist’s opinion.
The ECG parameters/intervals that were assessed at each visit were heart rate (beats/minute), PR, QRS, QT and QTc intervals (ms). The QT intervals were adjusted using Bazett’s corrections (QTcB). The mean/median QTc was calculated for each regimen at 0, 1, 2, 3 and 4 months. The stratified analysis of QTc at different time points with age, sex, body mass index (BMI), sputum smear grading and radiological involvement was done using SPSS version 20.0 software.
Results
Of the 602 patients enrolled, 476 were treated with the MFX regimens and 126 with the control regimen. The median age of the patients was 35 years, and 457 were men. The mean BMI was 16.3. The sputum culture grading was more than 2+ in 193 cases and 466 had more than 2-zone involvement on chest X-ray. The mean pre-treatment QTc was 403.90 and 403.54 ms in MFX and control regimen, respectively. There was no statistically significant increase in the mean QTc interval in either group of patients [Table - 1] up to 4 months. The median (IQR) of QTc in MFX regimen was 404.19 (389.83–416.71). However, in 5 patients, MFX had to be temporarily withheld for increase in the QTc interval above the upper limit described in the protocol (>450 ms) during treatment and MFX was re-introduced later without incident cardiac effects. In one patient, MFX was stopped on the advice of a cardiologist. The QTc was increased up to 487 ms and remained high on re-introduction of the drug. All other cardiac parameters were normal and there were no instances of arrhythmias.
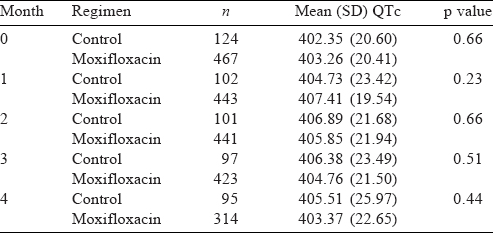
Stratified analysis of QTc at different time-points with age, sex, BMI, sputum smear grading and radiological involvement in both groups did not show any effect on QTc. The effect of MFX on other parameters such as heart rate, PR interval and QT interval at different time-points also showed no statistically significant difference in the control and MFX groups.
Discussion
The QT interval as recorded by a surface ECG is a measure of the time from the onset of ventricular depolarization to the end of ventricular repolarization (beginning of the QRS complex to the end of the T-wave). It quantifies the flow of ion currents across the cell membrane of ventricular myocytes through specialized protein channels. When these channels malfunction, they can disrupt normal cardiac rhythms and place a patient at risk of developing fatal cardiac arrhythmias. While most patients with prolonged QTc intervals do not go on to develop TdP, they are at a high risk of sudden cardiac death.[3] Hence, regulatory agencies have emphasized the importance of QTc assessment in the development of new drugs.[7] As most patients are asymptomatic, prolonged QTc is considered an indirect surrogate of predisposition to the induction of TdP.[8]
Prospective randomized studies have shown that MFX is related to an increased risk of QT prolongation. In a study of 18 healthy volunteers, a single dose of MFX 400 mg was shown to prolong the QT interval although the association with TdP was low,[9] whereas our analysis shows that in adults with pulmonary TB without any cardiac risk factors, MFX 400 mg given daily for up to 4 months did not cause an increase in the QTc interval.
The general consensus is that women are more sensitive to drug-induced QTc prolongation[10] compared to men, but our study shows a similar response in both sexes. Further, in our study, BMI did not influence QTc prolongation in contrast to the previously published literature.[11] There was no relation between mean QTc and age, baseline culture grading and lung zone involvement in chest X-ray.
One of the limitations of this study is the absence of simultaneous pharmacokinetic data to correlate blood levels of MFX with changes in QTc.
To conclude, our study indicates that MFX, at a dose of 400 mg given once daily, is well tolerated and without any adverse cardiac events.
Conflicts of interest. None declared
| 1. | Liu HH. Safety profile of the fluoroquinolones: Focus on levofloxacin. Drug Saf 2010;33:353-69. [Google Scholar] |
| 2. | Falagas ME, Rafailidis PI, Rosmarakis ES. Arrhythmias associated with fluoroquinolone therapy. Int J Antimicrob Agents 2007;29:374-9. [Google Scholar] |
| 3. | Harausz E, Cox H, Rich M, Mitnick CD, Zimetbaum P, Furin J, et al. QTc prolongation and treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2015;19:385-91. [Google Scholar] |
| 4. | Sides GD. QT interval prolongation as a biomarker for Torsades de pointes and sudden death in drug development. Dis Markers 2002;18:57-62. [Google Scholar] |
| 5. | Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577-87. [Google Scholar] |
| 6. | Velayutham B V, Allaudeen IS, Sivaramakrishnan GN, Perumal V, Nair D, Chinnaiyan P, et al. Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India. Clin Infect Dis 2014;59:e142-9. [Google Scholar] |
| 7. | Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist 2005;70:61134-5. [Google Scholar] |
| 8. | Malik M, Camm AJ. Evaluation of drug-induced QT interval prolongation: Implications for drug approval and labelling. Drug Saf2001;24:323-51. [Google Scholar] |
| 9. | Démolis JL, Kubitza D, Tennezé L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther 2000;68:658-66. [Google Scholar] |
| 10. | Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993;270:2590-7. [Google Scholar] |
| 11. | Alpert MA, Terry BE, Cohen MV, Fan TM, Painter JA, Massey CV, et al. The electrocardiogram in morbid obesity. Am J Cardiol 2000;85:908-10, A10. [Google Scholar] |
Fulltext Views
1,626
PDF downloads
10,120




