Translate this page into:
Effectiveness of education and antibiotic control programme at All India Institute of Medical Sciences, New Delhi
2 Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
3 Department of Biostatistics, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Naveet Wig
Department of Medicine, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
naveetwig@gmail.com
| How to cite this article: Ahmed SA, Kumar A, Sethi P, Kapil A, Pandey R M, Wig N. Effectiveness of education and antibiotic control programme at All India Institute of Medical Sciences, New Delhi. Natl Med J India 2018;31:262-267 |
Abstract
Background. We aimed to assess the impact of antibiotic optimization education along with understanding the antibiogram on antibiotic-prescribing practices, antibiotic consumption, antimicrobial resistance and cost of antibiotics in a tertiary care hospital in New Delhi.Methods. We divided the study into 3 phases—before and after intervention and a phase of education in between. We collected data on demographics, indication for antibiotic prescription, appropriateness or reasons for inappropriate antibiotic uses, antibiotic consumption (i.e. the rate and duration of antibiotic use), bacterial resistance and antibiotic cost. Interventions included education, introduction of an antibiogram and use of antibiotic prescription forms. Similar data were collected for the post-interventional phase. The study was conducted at the Department of Medicine, All India Institute of Medical Sciences, New Delhi, India.
Results. There was an improvement in the number of patients who underwent de-escalation of antibiotics, 21/100 v. 36/100 (p = 0.019); appropriate antibiotic usage, 25/ 100 v. 46/100 (p = 0.002); switching from intravenous to oral promptly, 16/52 v. 1 9/36 (p = 0.003) and decrease in expenditure, ₹24 207.5 v. ₹16 51 7.5 per patient (p = 0.001 ); in the post-interventional phase. Significant reductions in the incidence of infections due to Acinetobacter (60% v. 31%; p<0.001) and improvement in sensitivity pattern with cephalosporin sulbactam (80% v. 100%; p<0.001) were seen. Multivariate analysis revealed that Acute Physiology and Chronic Health Evaluation (APACHE) score, hospital stay <10 days, ventilator-associated pneumonia and methicillin-resistant Staphylococcus aureus coverage were independent predictors of mortality with odds ratio of 1.14, 0.1, 9.7 and 1.14, respectively.
Conclusion. Education and an antibiotic control programme constituted an effective and cost-saving strategy to optimise antibiotic use at a tertiary care centre.
Introduction
Inappropriate antibiotic use is common and has led to increasing rates of antibiotic resistance among community-acquired and healthcare-related pathogens. Factors that contribute to antibiotic overuse include lack of education, inebriant response to patient’s expectations, past experience and incentives. Multifaceted interventions are needed to reduce unnecessary antibiotic use. Peer education and feedback to doctors on antibiotics can promote behavioural change. Almost all the WHO regions show >50% prevalence of antibiotic-resistant organisms. In this scenario, unfortunately, no national surveillance data are available on antimicrobial resistance from India.[1] Few studies from India have shown that the prevalence of antibiotic resistance to various organisms ranges from 50% to 90%.[2] Health organizations should develop policies to support judicious antibiotic use and evaluate whether the existing policies are unintentionally promoting overuse of antibiotics.[3] This strategy aims at minimizing any unnecessary, inappropriate or irrational use of antimicrobials.
Construction of an antibiogram helps in providing appropriate antimicrobial therapy to all patients presenting with sepsis, thereby saving human lives. This study observed the pattern of antibiotic prescription with special reference to de-escalation, quantifying antibiotic usage and expenditure for antibiotics, switching from intravenous to oral antibiotics, provision of an antibiogram and to examine the impact of educational programmes and antibiogram on the outcomes in sepsis syndromes.
Methods
The study was conducted at the Medicine Intensive Care Unit (ICU) and wards of the Department of Medicine, All India Institute of Medical Sciences, New Delhi.
Design
The study was conducted in 3 phases—phase 1 : observation of current practices; phase 2: intervention in the form of feedback on current practices and educational sessions on ideal practices with provision of an antibiogram; and phase 3 : observation of change in practices. It was conducted between March 2013 and September 2014 with a sample size of 100 patients each for phase 1 and phase 3. In phase 1 of 9 months (March–November 2013), all episodes of systemic inflammatory response syndrome (SIRS) treated over a 9- month period in the medical wards and ICU were reviewed. Appropriateness or inappropriateness of initial antimicrobial therapy, de-escalation, escalation, no change and mixed changes in the antibiotic regimen were documented based on tools mentioned in the study. In phase 2 of 3 months (December 2013–February 2014), the findings from phase 1 were analysed and feedback was given to the residents on the current antibiotic practices. Antibiogram, interactive educational sessions and forums dedicated to antibiotic stewardship through Facebook and WhatsApp were made for the resident doctors. In phase 3 of 6 months (March 2014–September 2014), all data similar to phase 1 were noted. Changes in the antibiotic practices were observed and analysed.
Definitions
The rate of antibiotic use by inpatients was recorded in grams of the drug, and the value was converted into defined daily doses (DDDs) per 1000 patient-days, in accordance with WHO recommendations. An antibiogram is a periodic summary of antimicrobial susceptibilities of local bacterial isolates submitted to the hospital’s clinical microbiology laboratory. It serves as a tool to inform clinicians about local antibiotic susceptibility rates, aiding in choosing empirical antibiotic therapy and in monitoring resistance trends over time within an institution based on the latest Clinical and Laboratory Standards Institute (CLSI) guidelines.[4],[5] Antibiotic stewardship programme was defined as the optimal selection, dosage and duration of antimicrobial treatment that results in the best clinical outcome for the treatment or prevention of infection, with minimal toxicity to the patient and minimal impact on subsequent resistance,[6] based on guidelines by the Centers for Disease Control,[7] USA, and the Society for Healthcare Epidemiology of America in collaboration with the Infectious Diseases Society of America.[8] A major boost in India’s efforts towards antibiotic stewardship was brought about by the Chennai Declaration.[9],[10] In an effort to draw a roadmap to prevent antibiotic resistance, patients were assessed daily till hospital discharge or mortality. These tools were used, in our study, to scale the appropriateness of antibiotics. We used disease severity scores at hospital admission such as Simplified Acute Physiology Score (SAPS III) and Acute Physiology and Chronic Health Evaluation (APACHE II) scores.
Statistical analysis
Categorical variables were presented as absolute values, and percentages were compared using chi-square or Fisher’s exact test, as appropriate. For continuous variables, mean, standard deviation and median were calculated. Statistical analysis was done using STATA version 11 software. Continuous variables with parametric data were compared using t-test and non-parametric data were compared using the Wilcoxon analysis. Categorical variables were compared using the chi-square/Fisher’s exact test. Variables related to the dependent outcome with p<0.2 in univariate analysis were included in multivariable analysis. A value of p<0.05 was considered statistically significant in multivariable analysis.
Results
Demographic profile
In both phase 1 and phase 3, 100 patients were included. Of these 100 patients, 25 patients each were included in SIRS, sepsis, severe sepsis and septic shock groups in both the phases. In phase 1, 30 patients were admitted to the ICU and in phase 3, 21 patients were admitted to the ICU (p=0.144). There was no significant difference in age or sex distribution between the 2 phases [Table - 1],[Table - 2],[Table - 3].{Table 1}{Table 2}{Table 3}
The distribution of comorbid conditions such as hypertension, diabetes, chronic obstructive pulmonary disease (COPD), coronary artery disease, cerebrovascular accident, chronic kidney disease, congestive cardiac failure, malignancy and immune-suppressed (40 mg prednisolone equivalent for a minimum of 3 months) had no significant differences between both phases. However, autoimmune diseases were significantly higher in phase 1 whereas haematological diseases were more in phase 3. There was no significant difference in the rates of smoking, alcohol consumption and tobacco chewing between phases.
Clinical and biochemical parameters
The patients in phase 3 had a significantly lower Glasgow Coma Scale and higher systolic blood pressure compared to those in phase 1. Phase 3 had a sicker cohort of patients (SAPS III, p=0.02); although on subgroup analysis, the difference was only seen in the SIRS and sepsis groups. However, patients in phase 1 had more hepatic dysfunction and hyperlactatemia. Among the groups, patients with sepsis had higher uric acid levels in phase 3. Patients with severe sepsis in phase 3 were more anaemic [Table - 1],[Table - 2],[Table - 3].
The source of infection was pneumonia (60%) followed by urinary tract infection and bloodstream infection without any significant difference in phases. There was a significant improvement in the number of blood cultures sent in the post-interventional phase, from 42% to 65% (p=0.001).
Antibiogram
Antibiogram based on the sensitivity patterns of isolates obtained from samples sent from medicine wards and ICU over a period of 5 months were made at the end of phases 1 and 3. The data regarding the sensitivity patterns were obtained from records in the Department of Microbiology. The antibiogram was made separately for tracheal aspirate, sputum, blood, urine and pus cultures. However, the change in the sensitivity patterns could not be analysed because of inadequate samples. As per the CLSI guidelines a minimum of 30 isolates are required for analysis. Acinetobacter, Klebsiella and Escherichia coli were the most common organisms grown in endotracheal aspirate, blood and urine cultures, respectively. In sputum samples, Klebsiella was the most common organism that was isolated. Among pus cultures, Pseudomonas was the most common in the pre-interventional phase, while Staphylococcus aureus was the most common in the post-interventional phase [Table - 4].{Table 4}
Pattern of antibiotic change
There was a significant improvement in the number of patients who underwent de-escalation in the post-interventional phase (p=0.019). Among the groups, a significant improvement was noted only in patients with SIRS and patients admitted in the ward. In about 25% of patients, there was no change of antibiotics, after starting empirical antibiotics [Table - 5]. Among patients who underwent de-escalation, about two-thirds of patients had a reduction in the number of antibiotics, whereas the rest had a reduction in the spectrum as compared to phase 1. In the post-interventional phase, over three-fourths of patients had a reduction in the number of antibiotics among those who underwent deescalation. However, this increase was not statistically significant.

Antibiotic usage and switching to oral antibiotics
There was a significant reduction in the amount of antibiotics used in the post-interventional phase (p=0.001). Among individual antibiotics, there was a significant reduction in the use of levofloxacin (p=0.0001), cefoperazone–sulbactam (p=0.004) and ceftriaxone (p=0.048). There was a significant increase in the use of amikacin in the post-interventional phase and improvement in the number of patients in whom antibiotics were switched from intravenous to oral drugs promptly (p=0.003, 16/52 in phase 1 v. 19/36 in phase 3) in the post-interventional phase, even though there was a significant reduction in the number of patients who satisfied the conditions for oral switching.
Appropriate antibiotic usage
There was a significant improvement in the appropriate antibiotic usage in the post-interventional phase (p=0.002). However, this was not seen in the septic shock group [Table - 6].

Outcome parameters
There was a significant reduction in the amount of money spent on antibiotics in the post-interventional phase (p=0.013). There was a significant increase in the ICU mortality (p=0.047), which can be attributed to the presence of more sick patients in the post-interventional phase. However, there was no difference in the overall mortality rate. The mortality rates in sepsis, severe sepsis and septic shock, in phases 1 and 3, were 16%, 36% and 60%, and 20%, 48% and 60%, respectively [Table - 7] and [Table - 8].{Table 7}{Table 8}
Predictors of mortality
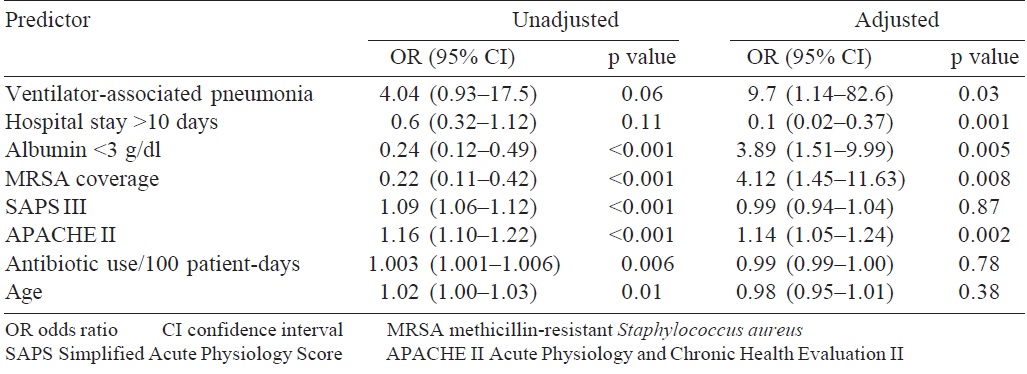
On comparing the survival and mortality groups, patients with pneumonia, COPD and positive cultures had higher mortality rates. Patients admitted to ICU and patients with higher SAPS III and APACHE II scores had higher mortality rates. Older patients had higher mortality rates. Mortality was higher in patients, who had creatinine >1.5 g/dl, abnormal serum glutamic pyruvic transaminase, acidosis, hypoalbuminaemia, hyperlactatemia and prothrombin time >17 seconds. Those patients who received antibiotic cover for methicillin-resistant Staphylococcus aureus (MRSA), fluoroquinolones and increased clindamycin use had higher mortality rates. Multivariate analysis revealed APACHE II, hospital stay <10 days, ventilator-associated pneumonia (VAP) and MRSA cover as independent predictors of mortality. Each unit increase in APACHE II score was associated with 1.14 times increase in mortality (odds ratio [OR] 1.14; 95% CI 1.05–1.24). MRSA coverage was associated with 4.12 times increase in mortality rate (OR 4.12; 95% CI 1.45–11.6). Hospital stay >10 days was associated with 0.1 times increase in mortality rate, i.e. shorter hospital stay was associated with increased mortality. This may be due to the early mortality in severe sepsis and septic shock (OR 0.1 ; 95% CI 0.02–0.37). The presence of VAP was associated with 9.7 times increased risk of mortality (OR 9.7; 95% CI 1.14–82.6; [Table - 9]).
Discussion
Improving practices of antimicrobial use in hospitals is a complex and challenging task. Various approaches taken in developed countries include educational programmes, development of a restricted hospital formulary, limitations on reports of sensitivity tests, regulation of interactions between pharmaceutical representatives and physicians, controlled distribution, automatic stop-orders and written justification for specific antimicrobial agents and/or requirement for expert approval before or after prescribing some medications. Although several studies have focused on reduction in antibiotic volume and cost, few have documented the effect of such interventions on the appropriateness of antibiotic use.[11] Our study shows that an easily applicable, inexpensive, multifaceted educational programme was highly effective in a tertiary care hospital in a developing country. The intervention had an evident impact on prescribing practices, antibiotic use rates, bacterial resistance and cost-savings over 1 year.
The mean age of patients was comparable in the pre- and post-interventional phases and the sex distribution was almost equal in the pre-interventional phase, whereas the number of men slightly outnumbered women in the post-interventional phase (60% v. 40%, respectively). However, the difference was not statistically significant. There was a significant improvement in the number of blood cultures sent in the post-interventional phase (42% v. 65%, respectively, p=0.001), which signifies the impact of the educational programme in the management of sepsis. Combination of 2 or 3 antibiotics was the most prevalent prescription pattern in both the phases (almost 80%). The combination of piperacillin–tazobactam with azithromycin was used more frequently in phase 3 instead of levofloxacin in phase 1. This was a notable improvement, as increased use of quinolones has been shown to increase the emergence of resistant organisms and multi drug-resistant tuberculosis.[12] This trend is similar to that observed by El-Solh et al.[13] who analysed antibiotic-prescribing pattern in patients of nosocomial pneumonia, admitted to 3 tertiary care centres. Fluoroquinolones (51.4%), ceftriaxone (45%) and azithromycin (42.15%) were the 3 most commonly used antibiotics.
After the intervention, there was a significant improvement in the number of patients (21/100 v. 36/100) who underwent deescalation, amount of antibiotic used (p=0.001) and appropriate antibiotic (25/100 v. 46/100; p=0.002). This was the primary purpose of the study. The evidence basis of de-escalation is strong. A randomized controlled trial by Leone et al.,[14] the first trial on this topic, had similar findings. There is further scope for improvement to master the art of de-escalation. Bhakta et al.[15] and Bajpai and Karnad[16] have shown that de-escalation is associated with reduced mortality, shorter duration of hospital stay and savings on expenditure on antibiotics. In a study by Hadi et al.[11] done in medicine wards in 5 hospitals in Indonesia, post-educational intervention antibiotic usage reduced from 99 DDD/100 patient-days to 71 DDD/100 patient-days. Our antibiotic usage was still too high, though there was a significant reduction in its use.
There was a significant improvement in the number of patients in whom antibiotics were switched from intravenous to oral drugs promptly in the post-interventional phase (16/52 v. 19/36; p=0.003), improvement in appropriate antibiotic use (25/100 v. 46/100; p=0.002) and reduction in the amount of money spent on antibiotics (₹24 201.5 v. ₹16 511.5; p=0.013). A study by Badar and Navale[18] in a tertiary care teaching hospital in Central India revealed that appropriate antibiotic use was only 30%.
A multivariate analysis revealed APACHE II, hospital stay <10 days, VAP and MRSA coverage as independent predictors of mortality, with OR of 1.14, 0.1, 9.7 and 1.14, respectively. There were significant reductions in the incidence of infections due to Acinetobacter (60% v. 31%; p<0.001) and also in sensitivity pattern with cephalosporin sulbactam (80% v. 100%; p<0.001). A study done by Boussekey et al.[19] for 5 years in an ICU in France to look for independent predictors of mortality concluded that mechanical ventilation, SAPS II >60, chronic alcoholism, age >65 years and prothrombin ratio <40% are independent predictors of mortality. Another study done at our institute by Prajowl Shrestha[20] also looked for predictors of mortality in ICU patients with sepsis. This study revealed that anaemia, SAPS II score >35 and SAPS III score >47 were independent predictors of mortality.
Conclusion
A significant improvement was seen in de-escalation practices in the post-interventional phase. De-escalation leads to a reduction in the duration of hospital stay, cost of antibiotics and reduction in antibiotic use. Improvement was seen in the number of blood cultures that were sent in cases of sepsis, in the post-interventional phase. APACHE II and SAPS III scores, appropriate initial antibiotic use, serum albumin levels, hospital stay <10 days and VAP and MRSA coverage were found to be independent predictors of mortality. Our study emphasizes the importance of antibiotic stewardship programmes, its periodic review and maintenance in preventing emergence of multi drug-resistant organisms.
Conflicts of interest. None declared
| 1. | WHO. Antimicrobial resistance: Global Report on Surveillance. WHO; 2014. Available at www.who. int/drugresistance/documents/surveillancereport/en/ (accessed on 4 Nov 2014). [Google Scholar] |
| 2. | Kumar SG, Adithan C, Harish BN, Suj atha S, Roy G, Malini A, et al. Antimicrobial resistance in India: A review. J Nat Sci Biol Med 2013;4:286-91. [Google Scholar] |
| 3. | Belongia EA, Schwartz B. Strategies for promoting judicious use of antibiotics by doctors and patients. BMJ 1998;317:668-11. [Google Scholar] |
| 4. | Joshi S. Hospital antibiogram: a necessity.Indian J Med Microbiol 2010;28:211-80. [Google Scholar] |
| 5. | Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the clinical and laboratory standards institute. Clin Infect Dis 2007;44:867-73. [Google Scholar] |
| 6. | Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096. [Google Scholar] |
| 7. | Core Elements—Implementation Resources—Get Smart for Healthcare—CDC. Available at www.cdc.gov/getsmart/healthcare/implementation/core-elements.html (accessed on 9 Nov 2014). [Google Scholar] |
| 8. | Society for Healthcare Epidemiology of America. Infectious Diseases Society of America. Paediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Paediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012;33:322-7. [Google Scholar] |
| 9. | Chennai Declaration. Available at www.chennaideclaration.org/ (accessed on 9 Nov 2014). [Google Scholar] |
| 10. | Holmes AH, Sharland M. The Chennai declaration: India’s landmark national commitment to antibiotic stewardship demonstrates that ‘truth alone triumphs’. J Antimicrob Chemother 2013;68:1453-4. [Google Scholar] |
| 11. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Google Scholar] |
| 12. | Thabet L, Memmi M, Turki A, Messadi AA. The impact of fluoroquinolones use on antibiotic resistance in an intensive care burn department. Tunis Med 2010;88: 297-300. [Google Scholar] |
| 13. | El-Solh AA, Peter M, Alfarah Z, Akinnusi ME, Alabbas A, Pineda LA. Antibiotic prescription patterns in hospitalised patients with nursing home-acquired pneumonia. J Hosp Med 2010;5:E5-10. [Google Scholar] |
| 14. | Leone M, Bechis C, Baumstarck K, Lefrant JY, Albanése J, Jaber S, et al. Deescalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multi-center non-blinded randomised non inferiority trial. Intensive Care Med 2014;40:1399-408. [Google Scholar] |
| 15. | Bhakta A, Bhattacharya M, Todi S. De-escalation practice pattern in an Indian intensive care unit. Crit Care 2010;14 Suppl 1:P63. [Google Scholar] |
| 16. | Bajpai S, Karnad DR. De-escalation of antibiotics in nosocomial pneumonia in an Indian intensive care unit. Int J Med Med Sci 2010;2:148-52. [Google Scholar] |
| 17. | Hadi U, Keuter M, van Asten H, van den Broek P, Study Group ‘Antimicrobial resistance in Indonesia: Prevalence and Prevention’ (AMRIN). Optimising antibiotic usage in adults admitted with fever by a multifaceted intervention in an Indonesian governmental hospital. Trop Med Int Health 2008;13:888-99. [Google Scholar] |
| 18. | Badar VA, Navale SB. Study of prescribing pattern of antimicrobial agents in medicine intensive care unit of a teaching hospital in central India. J Assoc Physicians India 2012;60:20-3. [Google Scholar] |
| 19. | Boussekey N, Cantrel J, Dorchin Debrabant L, Langlois J, Devos P, Meybeck A, et al. Epidemiology, prognosis, and evolution of management of septic shock in a French intensive care unit: a five years survey. Crit Care Res Pract 2010;2010:436427. [Google Scholar] |
| 20. | Prajowl Shrestha AM. To determine the predictors of mortality and morbidity of sepsis in medical ICU of all India Institute of Medical Sciences (AIIMS), New Delhi, India. Chest 2012;142 4_Meeting Abstracts:407A. [Google Scholar] |
Fulltext Views
2,549
PDF downloads
556




