Translate this page into:
Effectiveness of symptom screening and incidence of tuberculosis among adults and children living with HIV infection in India
2 Government Vellore Medical College and Hospital, Vellore, Tamil Nadu, India
3 Government Rajaji Medical College and Hospital, Madurai, Tamil Nadu, India
4 Ashakirana Hospital, Mysore, Karnataka, India
5 Government Kilpauk Medical College and Hospital, Kilpauk, Tamil Nadu, India
6 Government Headquarters Hospital, Krishnagiri, Tamil Nadu, India
7 Niloufer Hospital, Hyderabad, Telengana, India
8 Government District Hospital, Tiruvallur, Tamil Nadu, India
9 Indira Gandhi Institute of Child Health, Bengaluru, Karnataka, India
10 National Institutes of Tuberculosis and Lung Diseases, New Delhi, India
11 Institute of Child Health and Children's Hospital, Chennai, Tamil Nadu, India
12 National AIDS Control Organization, New Delhi, India
Corresponding Author:
C Padmapriyadarsini
National Institute for Research in Tuberculosis, No. 1, Sathiyamoorthy Road, Chetput, Chennai 600031, Tamil Nadu
India
pcorchids@gmail.com
| How to cite this article: Padmapriyadarsini C, Bhavani P K, Sekar L, A, Selvaraj M, Poornagangadevi N, Mothi S N, Nandagopal K, Vennila S, Priyadarshini G K, Manisha M, Sanjeeva G, Agarwal U, Suresh E, Rewari B B, Swaminathan S. Effectiveness of symptom screening and incidence of tuberculosis among adults and children living with HIV infection in India. Natl Med J India 2016;29:321-325 |
Abstract
Background. WHO recommends the use of a simplified symptom-based algorithm for screening for tuberculosis (TB) among people living with HIV (PLHIV). We assessed the feasibility and effectiveness of this algorithm and determined the prevalence and incidence of TB among PLHIV attending antiretroviral treatment (ART) centres in India.Methods. We did a prospective multicentric implementation research study in four states of India. To rule out TB, we administered the WHO symptom-screen algorithm to all PLHIV every month for 6 months. If they were found to be symptomatic any time during this period, they were referred for investigations for TB. A case of TB diagnosed during the first month of screening was taken as a prevalent case while those detected TB in the subsequent 5 months were considered cases of incident TB. We calculated the incidence rate using the person-years method.
Results . Between May 2012 and October 2013, a total of 6099 adults and 1662 children living with HIV were screened for TB at the ART centres of four states. Of the 6099 adult PLHIV, 1815 (30%) had at least one symptom suggestive of TB, of whom only 634 (35%) were referred for investigations of TB. Of those referred, 97 (15%) PLHIV were diagnosed with TB. Overall, the prevalence of undiagnosed TB was 0.84 person-years and in the subsequent period, the incidence of TB was 2.4/100 person-years (95% CI 1.90-3.10). Among 1662 children, 434 (26%) had at least one symptom suggestive of TB. But only 57 (13%) children were referred for investigations of TB and 13 (23%) of them were diagnosed with TB. The prevalence of TB among children was 0.5% and its incidence among them was 2.7/100 person-years (95% CI 1.60-4.30).
Conclusion. Prevalence and incidence of TB is high among PLHIV attending ART centres. This emphasizes the need to strengthen regular screening for symptoms of TB and further referral of those symptomatic for diagnosis of TB.
Introduction
WHO estimates that of the 9 million people who developed tuberculosis (TB) in 2013, more than 1.1 million (13%) were HIV-positive. [1] The risk of developing TB among people living with HIV (PLHIV) is 20-37 times higher compared with HIV-uninfected people. [1] Even in the era of antiretroviral therapy ( art0 ), TB remains the leading cause of morbidity and mortality among PLHIV. [2] Hence, it is necessary to take steps to reduce the risk of developing HIV-associated TB. WHO currently recommends ART and its strategy of three I′s-intensified case-finding (ICF), isoniazid preventive therapy (IPT) and infection control to reduce the risk of HIV-associated TB. [3] In 2011, WHO released simplified guidelines for ICF and IPT, recommending that all PLHIV should be regularly screened for TB using a clinical algorithm of any cough, night sweats, weight loss and/or fever (and failure to thrive in children). However, regular screening for TB of all PLHIV at every visit to an ART centre is not routinely done in many countries with a high burden of TB.
India accounted for 24% of the total 9 million cases of TB in 2013. [1] TB is estimated to cause about 25% of all deaths among PLHIV in India. [4] Hence, it is critical for India to adopt WHO′s key interventions, namely ICF and IPT, to reduce mortality secondary to HIV-associated TB. The National AIDS Control Programme decided, in principle, to implement IPT as part of its package of interventions but wanted to determine the feasibility and effectiveness of WHO′s symptom algorithm to rule out TB as part of ICF. Hence, we assessed the utilization, feasibility and effectiveness of a simple clinical algorithm to exclude TB in both high and low TB burden centres and estimated the prevalence and incidence of TB among adults and children living with HIV.
Methods
We did a prospective multicentre study to measure the prevalence and incidence of TB and the effectiveness of symptom screening to exclude TB among adults and children attending 10 ART centres in four states of India. The participating ART centres were selected on the basis of their patient load, presence of a medical officer (MO) experienced in the management of HIV-infected and TB patients, facilities for free chest X-ray and adequate human resources for close supervision of patients on treatment and follow-up. At these sites, we carried out enhanced TB surveillance (i.e. provider-initiated active screening for TB) for 6 months using WHO′s symptom-based screening algorithm.
HIV-infected adults and children, attending these ART centres, were eligible to participate in the study (i) if they had documented evidence of HIV infection; (ii) if they were older than 2 years of age; (iii) if they had not received treatment for TB or IPT in the previous year; and (iv) if they were willing to adhere to the study procedures and follow-up schedule as required. Patients with current evidence of TB disease (as assessed by the ART MO) and currently on anti-TB treatment (ATT) or IPT were not enrolled in the study.
On entry to the study, and after obtaining written informed consent (assent from children wherever applicable), we administered WHO′s standardized TB symptom screen programme to PLHIV. Presumptive TB was considered if a patient had one or more of the following symptoms: any (current) cough, fever, drenching night sweats or unintentional weight loss (additionally, failure to thrive in children). The symptom screening was done by trained clinic staff other than the MO. We offered counselling on cough hygiene to symptomatic patients, and referred them to the ART MO for further clinical evaluation. The ART MO referred these patients for further diagnostic testing, usually by means of sputum microscopy and chest X-ray as per the Revised National Tuberculosis Control Programme (RNTCP) guidelines. Diagnosis of pulmonary TB among adults was based on clinical history and physical examination, chest X-ray and sputum smear microscopy, while in children it was mostly a clinical and radiological diagnosis with expectorated sputum sample wherever feasible. Extrapulmonary TB was diagnosed by either chest X-ray, fine-needle aspiration cytology (FNAC) or tissue biopsy. Culture for acid-fast bacilli (AFB) was not done as it is not part of the investigations for TB in the RNTCP guidelines. Those who were smear-positive and those with unresolved symptoms at follow-up visits were referred to the RNTCP clinic for further management. All asymptomatic individuals were followed up for 6 months with the clinical symptom screen administered every month. A data collection card was specially designed for this study to capture the symptom screen every month, in which the staff at the centre assigned to do this work would record the symptoms or their absence at every visit. If patients had consulted doctors outside the government hospital and were investigated, then during their subsequent visit to the ART centre these details were recorded along with the results of the investigations, in the data collection card. This card was attached to the HIV/ART card of the patient so as not to miss any visit. The staff administering the symptom screen and the completion of the card was periodically supervised by the MO at the site and the study team.
During the follow-up visits, if the patient developed any of the above-mentioned symptoms, they were referred for evaluation of TB. All patients received the current standard of care during the 6 months of enhanced TB surveillance.
Those diagnosed with TB during the first month of screening were taken as prevalent cases, while those diagnosed in the subsequent 5 months were considered as cases of incident TB. At the end of 6 months of follow-up, the incidence rate along with confidence interval was calculated using the person-years method. Double data entry was done and all statistical analysis was carried out using the SPSS software 20.0 version.
The study was approved by the institutional ethics committee of the National Institute for Research in Tuberculosis, Chennai and all the 10 participating centres. Permission to conduct the study was also obtained from the respective state AIDS control societies (SACS) and the hospital scientific committee. Informed written consent was obtained from all patients before the commencement of the study.
Results
Between May 2012 and October 2013, a total of 6214 adults and 1690 children living with HIV consented to take part in the study at 10 ART centres in four states. Of these, 115 adults and 28 children had recently (i.e. within a year) completed a full course of ATT and thus were not eligible for participation in the study. Hence, the final enrolment had 6099 adults and 1662 children ([Figure - 1] and [Figure - 2]).
 |
| Figure 1. Schema of intensified screening for tuberculosis (TB) in adults attending an antiretroviral therapy (ART) centres |
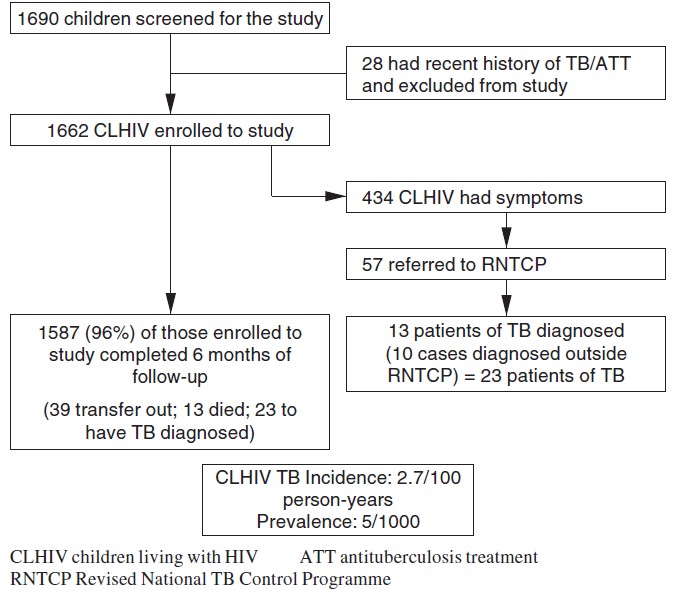 |
| Figure 2. Intensified screening for tuberculosis (TB) among children attending antivetroviral therapy (ART) centres |
Adults
The 6099 adult participants (52% women) had a mean (SD) age of 37.1 (11.7) years and their median CD4 cell count was 462 cells/cmm (IQR: 300-665). Three-fourths (74%; 4512) of individuals reported currently being on ART. During the 6 months of follow-up, 1815 of 6099 (30%) participants developed symptoms: 1239 (68%) individuals had one symptom while 576 (32%) had two or more symptoms suggestive of TB. Of the 1815 symptomatic individuals, only 634 (35%) were referred for investigations of TB; among these 97 (15%) individuals were diagnosed with TB. An additional 20 individuals were diagnosed with TB and started on ATT by healthcare practitioners outside the RNTCP.
Of the 117 (97+20) patients diagnosed with TB, 52 had extrapulmonary TB, 51 sputum smear-positive TB and 14 sputum smear-negative TB. Of these 117 patients, 52 (32 men and 20 women) were diagnosed within the first month of the study period. These patients were already in the community and came to light when they attended the ART OPD for either diagnosis of HIV or initiation of ART. This gave a prevalence of undiagnosed TB of 0.84% (52 of 6099). Thirty-seven of these 52 patients (71%) had CD4 <250 cells/cmm at the time of diagnosis. The remaining 65 patients were diagnosed between the second and sixth month of follow-up; these were considered as incident TB cases. The incidence of TB was 2.4/100 person-years (95% CI 1.90-3.10). The 65 new patients of TB (41 men and 24 women) indicated a higher TB incidence among men as compared to women (3.2 v.1.7/100 person-years); 48 were on ART while 17 were pre-ART (incident TB 2.2 v. 3.3/100 person-years, p=0.13), not statistically significant between the groups.
Children
Of the 1662 children living with HIV, 55% were boys, mean (SD) age of 9.6 (4.4) years and the median CD4 cell count was 813 cells/cmm (IQR 501-1186). Again, three-fourths of children (76%; 1269) were on ART; 434 of 1662 children (26%) were symptomatic: 325 (75%) had at least one symptom while 109 (25%) had two or more symptoms suggestive of TB. Of the 434 symptomatic children, only 57 (13%) were referred to RNTCP for investigations of TB; among whom 13 (23%) were diagnosed with TB. An additional 10 patients were diagnosed with TB and started on ATT by healthcare practitioners outside the RNTCP. Of the 23 TB patients (13 boys and 10 girls), 10 had smear-positive TB, 4 smear-negative and 9 extrapulmonary TB. Eight patients (3 boys and 5 girls) were diagnosed within the first month of the study period, while 15 (65%) (10 boys and 5 girls) were diagnosed between the second and sixth month of follow-up. Overall, the prevalence of undiagnosed TB was 0.47% and the incidence of TB was 2.7 per 100 person-years (95% CI 1.60-4.30). Of the 15 incident patients with TB, 11 were on ART while 4 were not (incident TB 1.8 v. 2.9/100 person-years, p=0.42).
Discussion
We found a high prevalence and incidence of TB among adults and children attending ART centres in four states of India, underscoring the importance of regular screening and diagnostic work-up for TB. Globally, PLHIV are 29 times (26-31) more likely to develop TB than those without HIV. [5] A previous study in this region found that the incidence of TB in HIV-infected adults was 7/100 person-years; and this was done in the pre-ART era. [6] While ART improves the immune system and reduces the incidence of TB, the risk of TB is still several-fold higher than that in HIV-negative persons.
In response to the dual epidemics of HIV and TB, WHO, in its 2011 guidelines, recommended the use of a simplified screening algorithm that relies on four clinical symptoms to identify those eligible for further diagnostic work-up for TB. According to these guidelines, PLHIV with any one of the symptoms of current cough, fever, weight loss or night sweats may have TB and should be evaluated further. [3] A meta-analysis reported that four TB-related symptoms (cough, fever, night sweats, weight loss) lacked the ability to identify PLHIV at extremely low risk for TB disease. [7] However, WHO adopted these screening criteria for TB case-finding among all PLHIV in 2011, [3] though it is still not being used universally to rule out TB in PLHIV. A study from Kenya found that <15% of PLHIV with symptoms suggestive of TB received any form of diagnostic evaluation [8] while in Ethiopia only 21% of PLHIV with chest symptoms were screened for TB. [9] In our study, only 35% of symptomatic patients were sent for further diagnostic evaluation of TB. The reasons for this were multifactorial from lack of referral by physicians to failure of patients to comply with the request. The commonest reasons for non-referral for investigations (from data collected through informal interview of staff) were short duration of cough (′this cough is not TB cough′), no history of contact, not associated with other symptoms, lack of staff, additional workload, etc. The distance of the TB laboratory from the ART centres, lack of availability of staff in the laboratory, transportation costs, etc. were few of the reasons for non-compliance of patients to go for work-up of TB. However, of those referred for investigations, almost a quarter were diagnosed with TB. This underlines the fact that the WHO′s screening algorithm is effective but it is not fully implemented.
Also, TB detection rates among the referred cases were low. The distance between the ART and RNTCP centres, delay in diagnosis of smear-negative or extrapulmonary TB, using sputum smear technique to diagnose TB at RNTCP centres, could be among the reasons for poor detection rates of TB among those referred. Using newer diagnostics such as cartridge-based nucleic acid amplification techniques (CB-NAAT) or Gene Xpert can increase the detection rate of TB among PLHIV. Placing integrated counseling and testing centres at RNTCP centres, early chest X-ray in the diagnostic algorithm of TB can reduce the delay in TB diagnosis and thus increase the TB detection rate. Also, individual screening and referral for the diagnosis of TB should be done not only at the time of diagnosis of HIV and initiation of ART but also during every follow-up visit. A study in Cape Town that investigated the effect of ART on the diagnostic utility of symptom screening to rule out TB among PLHIV showed a lower sensitivity (24% v. 48%) but a higher specificity (94% v. 80%) in the on-ART group compared with the pre-ART group. [10] Until more sensitive methods of ruling out TB are established, ongoing TB screening should continue using the WHO recommended tool at each visit.
Adopting a checklist approach to track whether a patient had been given the TB symptom screen and referred for further investigations for TB if the symptom screen was positive is a simple method to make sure that all symptomatic patients are referred for investigations. [11] Nurses or other paramedical staff can be trained to routinely perform effective TB screening as part of their duty, identify PLHIV with presumptive TB disease (i.e. those with a positive symptom screen) and refer them to the MO for diagnostic evaluation for TB. [12] ,[13] ,[14] Other interventions such as continuing medical education sessions, reminder posters, and the use of checklists can significantly improve rates of screening of TB among PLHIV.
As the cut-off level of CD4 for initiation of ART is being raised to 500 cells/cmm in India, more patients will be seen every month at earlier stages of HIV disease and the proportion to screen for TB will also increase. WHO recommends that Xpert MTB/RIF should be used rather than conventional microscopy as the initial diagnostic test for all persons with suspected HIV-associated TB. [15] As rapid molecular testing is being rolled out in the RNTCP, it will become more feasible and accessible to diagnose TB using these tests. Of the 9859 PLHIV screened in our cohort, only 6214 agreed to participate in the study. Around 36% of PLHIV were not willing to attend the ART centres monthly either because of distance from the clinic or they were pre-ART and were required to attend ART centres only once in 6 months; this is similar to studies from Botswana and Uganda. [16] ,[17] Expanding access to ART centres, TB laboratories and strengthening the collaboration between national AIDS and TB programmes can increase the proportion of PLHIV who can benefit from these programmes.
A major limitation of our study is that the vast majority of our cohort were PLHIV on ART and the representation of pre-ART PLHIV was limited in this population. Though we attempted to enrol all PLHIV attending ART centres, as per the current guidelines of the National AIDS Control Organization (NACO), pre-ART PLHIV are asked to attend ART centres only once in 6 months at the time of CD4 measurement or when they are symptomatic. They also get transferred to peripheral link ART centres. So, pre-ART PLHIV do not attend ART centres every month making it difficult to follow them up, as required. This could have led to a selection bias affecting the generalizability of our study. At the same time, it also raises the possibility that the actual incidence of TB may have been higher in the overall population of PLHIV. We report only on those who were symptomatic of TB and attended these ART centres and not on those who received care outside the government sector. The true prevalence/incidence may have been higher if all symptomatic patients had been evaluated. There is also a small possibility of misclassification, based on our definition of prevalent and incident cases. Rarely, TB may be missed during baseline screening and a PLHIV be started on ART. They may develop TB IRIS, manifesting at the second or third month and be counted as incident TB. This could have increased the incidence of TB. This over-emphasizes the need to screen all PLHIV at every visit for TB, not only with a symptom screen, but if found positive to subject those to more sensitive diagnostic tests to rule out TB. Further, some of the deaths (which mostly happened at home) could have been due to TB, further adding to the underestimation.
Conclusion
Prevalence and incidence of TB is high among PLHIV including those on ART. The WHO′s screening algorithm is effective but it is not fully implemented as not all symptomatic patients are being referred for investigations for TB. Thus, there is a need to strengthen screening and referral for diagnosis of TB. All symptomatic PLHIV should be screened for TB not only at the time of diagnosis of HIV and initiation of ART but also during every follow-up visit. Healthcare providers should be periodically trained at ART centres. To improve screening and diagnosis of TB in this vulnerable population, there is a need for better coordination with RNTCP as well as for more sensitive diagnostic processes.
Acknowledgements
We thank the staff of the Epidemiology Unit of the National Institute for Research in Tuberculosis (Mr V. Partheeban, Mr Sargunan, Mr Nagaraj, Mr Premkumar and Mr Ramesh), Department of Biostatistics (Mr Tamilchelvan), Department of Clinical Research (Mrs Gunasundari) and all members of the participating ART centres. We also thank the senior staff of NACO and the Central TB Division for their support and cooperation in the conduct of the study. We extend our sincere gratitude to all the study participants and their family members.
Contributions
CP, PKB, NP, LS, SS: protocol preparation, conduct of study, data analysis and manuscript preparation
AC, MS, KN, SNM, SV, MM, GS, GKP, UA, ES: participant enrollment, follow-up and data collection
BBR: protocol and manuscript preparation
Conflict of interest: None declared
| 1. | World Health Organization. Global Tuberculosis Report 2014. WHO/HTM/TB/2014.08. Geneva, Switzerland:WHO; 2014. Available at www.who.int/tb/publications/global_report/gtbr14_main_text.pdf?ua=1Global tuberculosis (accessed on 1 Feb 2015). [Google Scholar] |
| 2. | Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med 2009; 30: 685-99, viii. [Google Scholar] |
| 3. | World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2011. Geneva, Switzerland:WHO; 2011. Available at http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf?ua=1 (accessed on 1 Feb 2015). [Google Scholar] |
| 4. | National Framework for Joint HIV-TB Collaborative Activities, 2013. Central TB Division, Directorate General of Health Services and Basic Services Division, Department of AIDS Control, Ministry of Health and Family Welfare, Government of India, New Delhi. [Google Scholar] |
| 5. | HIV associated tuberculosis: Challenges and key issues. Geneva:World Health Organization; 2014. Available at www.who.int/tb/challenges/hiv/tbhiv_factsheet_2014.pdf (accessed on 18 Aug 2015). [Google Scholar] |
| 6. | Swaminathan S, Ramachandran R, Baskaran G, Paramasivan CN, Ramanathan U, Venkatesan P, et al. Risk of development of tuberculosis in HIV-infected patients. Int J Tuberc Lung Dis 2000; 4: 839-44. [Google Scholar] |
| 7. | Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: Individual participant data meta-analysis of observational studies. PLoS Med 2011; 8: e1000391. [Google Scholar] |
| 8. | Burmen B, Modi S, Cavanaugh JS, Muttai H, McCarthy KD, Alexander H, et al. Tuberculosis screening outcomes for newly diagnosed persons living with HIV, Nyanza Province, Kenya, 2009. Kenya, July 2009-August 2010. Proceedings of the 19th International AIDS Conference: Abstract no. MOPE650. 22-27 July 2012, Washington, DC: USA. [Google Scholar] |
| 9. | World Health Organization. Global Tuberculosis Report: 2012. Contract No: WHO/HTM/TB/2012.6. France:WHO;2012. [Google Scholar] |
| 10. | Rangaka MX, Wilkinson RJ, Glynn JR, Boulle A, van Cutsem G, Goliath R, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis 2012; 55: 1698-706. [Google Scholar] |
| 11. | Zaeh S, Kempker R, Stenehjem E, Blumberg HM, Temesgen O, Ofotokun I, et al. Improving tuberculosis screening and isoniazid preventive therapy in an HIV clinic in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis 2013; 17: 1396-401. [Google Scholar] |
| 12. | Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): A pragmatic, parallel, cluster-randomised trial. Lancet 2012; 380: 889-98. [Google Scholar] |
| 13. | Zachariah R, Ford N, Philips M, Lynch S, Massaquoi M, Janssens V, et al. Task shifting in HIV/AIDS: Opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg 2009; 103: 549-58. [Google Scholar] |
| 14. | Date A, Modi S. TB screening among people living with HIV/AIDS in resource-limited settings. J Acquir Immune Defic Syndr 2015; 68 Suppl 3:S270-3. [Google Scholar] |
| 15. | World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. WHO/HTM/TB/2013.16. Geneva:WHO; 2013. [Google Scholar] |
| 16. | Mosimaneotsile B, Mathoma A, Chengeta B, Nyirenda S, Agizew TB, Tedla Z, et al. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: A Botswana experience, 2004-2006. J Acquir Immune Defic Syndr 2010; 54: 71-7. [Google Scholar] |
| 17. | Mugisha B, Bock N, Mermin J, Odeke RM, Miller B, Adatu-Engwau F, et al. Tuberculosis case finding and preventive therapy in an HIV voluntary counseling and testing center in Uganda. Int J Tuberc Lung Dis 2006;10:761-7. [Google Scholar] |
Fulltext Views
1,720
PDF downloads
1,665




