Translate this page into:
Evaluation of cervical cancer screening during pregnancy in India: Human papillomavirus testing can change the paradigm
Correspondence to NEERJA BHATLA; neerja.bhatla07@gmail.com
[To cite: Sudhakaran S, Bhatla N, Mathur SR, Mahey R, Vashist S, Natarajan J, et al. Evaluation of cervical cancer screening during pregnancy in India: Human papillomavirus testing can change the paradigm. Natl Med J India 2023;36:17–21. DOI:10.25259/NMJI_768_20]
Abstract
Background
The World Health Organization’s call for elimination of cervical cancer envisages 70% screening coverage of women aged 35 and 45 years by an effective test. In India, this target seems unrealistic as awareness and access to cancer prevention services are poor. However, the institutional delivery rate is now >80%. We evaluated the acceptability and feasibility of human papillomavirus (HPV) testing and its role in screening during pregnancy.
Methods
This observational study recruited 275 pregnant women aged >25 years between 12 and 34 weeks of gestation for screening by cytology and HPV testing. Colposcopy was advised if either test was positive. Acceptability and feasibility were assessed by a questionnaire.
Results
Cytology and HPV reports were available for 269 subjects. The median age was 28 years and median parity was two. Only 98 (36.4%) had heard about carcinoma cervix. Awareness improved with education (p<0.001). On cytology, only 4 (1.5%) were abnormal (atypical squamous cells of undetermined significance 3; low-grade squamous intraepithelial lesion 1). The prevalence of high-risk HPV infection was 8.2% (22/269). On colposcopy, all had the Swede score <5. No high-grade cervical intraepithelial neoplasia or carcinoma was detected. Pre-procedure, 183 (68.0%) subjects expressed apprehension, post-procedure 114 (42.4%) of them had realized that their apprehensions were unfounded. Women found screening to be more uncomfortable after 28 weeks of gestation (n=26/68; 38.2%; p<0.001). Physicians found the cervix more difficult to visualize after 20 weeks of gestation (p<0.001).
Conclusions
HPV screening at 16–20 weeks of pregnancy is acceptable, feasible, and can greatly improve screening coverage in resource-limited settings. Pregnancy is a good opportunity to improve awareness of the screening programmes.
INTRODUCTION
Cervical cancer is the second-most common cancer among women in India, after breast cancer.1 Due to its long pre-invasive state, screening plays an important role in prevention of cervical cancer.2 However, in India, there is poor access to healthcare services and the antenatal period is often the only time when women are likely to come in contact with a healthcare provider. The All India Hospital Postpartum Programme by the Ministry of Health and Family Welfare, Government of India, initiated cervical cytology screening during pregnancy in the 1980s but it did not prove to be a successful strategy as a low proportion (15%) of women delivered in facilities at that time, most were young, with no or low-grade abnormalities, and quality control was poor. It was eventually discontinued.3 Visual inspection with acetic acid during pregnancy was tried subsequently, but it was difficult to interpret colour changes on an already congested cervix and there was a high false-positive rate.4,5
In recent years, human papillomavirus (HPV) testing has been found to be the most sensitive test for detection of cervical intraepithelial neoplasia (CIN).6 Primary HPV screening is recommended for use for the age group of 25–30 years by most guidelines, depending on the type of test used and resource.7–9 In 2019, the WHO announced the call to action for the elimination of cervical cancer by 2030, which envisages testing all women at age 35 and 45 years with an HPV test.10 Meanwhile, considerable political will has resulted in an increase in institutional deliveries to over 80%.11 Also, with increasing empowerment of women, there has been an increase in age at marriage and first pregnancy.
We evaluated acceptability, feasibility and efficacy of HPV testing during pregnancy, utilizing the antenatal visit as an opportunity to test the knowledge and improve awareness of women regarding cervical cancer, to detect the prevalence of abnormal cervical cytology and HPV infection among pregnant women, and to evaluate the possible role of screening during pregnancy to augment the national screening programme in India and other low- and middle-income countries (LMICs).
METHODS
This prospective observational study was done in the Antenatal Clinic from August 2016 to May 2018. The study was approved by the Institutional Ethics Committee of the All India Institute of Medical Sciences, New Delhi. Women aged >25 years, between 12 and 34 weeks of gestation, sexually active for >3 years, and willing to participate were enrolled in the study. Exclusion criteria were screening within the past 3 years, history of cervical incompetence, ultrasound showing major degree of placenta previa, and active infections/bleeding involving cervix/ vagina.
Informed written consent was obtained, and clinical history and examination findings, including obstetric examination, were recorded. Conventional Pap smear was obtained using Ayre’s spatula and endocervical brush. The endocervical brush used in conventional Pap smear is a soft, narrow cylindrical nylon brush which samples only the lower half of the endocervical canal. The Digene® sampling device (Qiagen Inc., Germany) was next used to collect a cervical sample in the Digene Specimen Transport Medium. HPV DNA testing was done by the Digene HPV test which detects 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). A cut-off ratio of >1.0 relative light units (RLU) was considered abnormal. Colposcopy was advised if either test was abnormal, and lesions were scored using the Swede score.12 A Swede score of >5 was considered the risk threshold for determining high-grade lesions (CIN2+).13 Cervical biopsy was obtained from all lesions using a fine Tischler forceps in all cases with a Swede score >2 to accurately identify all grades of cervical abnormalities. Endocervical curettage was avoided. Women with abnormal test reports received management as per the standard of care and counselling regarding implications for future follow-up.
Pre- and post-test questionnaires were completed to assess knowledge, attitude and practice of cervical cancer screening. All participants were informed about the screening protocols and encouraged to continue routine screening.
Data were analysed using STATA version 15.0. To determine the association between categorical variables, Chi-square/ Fischer exact test were applied as appropriate, and a value of p <0.05 was considered statistically significant. Descriptive statistics such as mean, standard deviation and range were calculated for normally distributed data.
RESULTS
Of 278 antenatal women invited to participate in the study, 275 agreed, and complete data were available for 269 women. Only 32 (11.9%) were illiterate while 105/269 (39.0%) were graduates. The mean (SD) age of the study subjects was 28.7 (3.8) years, mean age at coitarche was 22.5 (3.7) years (range 11–34 years), with the majority (n=172, 63.9%) between 18 and 24 years of age. Most (97.4%) were asymptomatic. A few presented with complaints, for example, breast lump (n=1), vaginal discharge (n=3), and spotting per vaginum (n=3) associated with threatened abortion. Three of them had received HPV vaccination previously.
Pap smear abnormalities
On examination, there was no obvious abnormality in any patient. Adequate smears were obtained in 265 (98.5%) women. Four (1.5%) had abnormal smears: atypical squamous cells of undetermined significance (ASCUS; 3) and low-grade squamous intraepithelial lesion (1). There was no high-grade squamous intraepithelial lesion or invasive carcinoma. Additional features were reported in 149 subjects, but none of these obscured the evaluation and interpretation of the smears: Inflammation (n=131, 50.2%), cytolysis (n=12, 4.6%), squamous metaplasia (n=3, 1.2%), trophoblastic giant cell (n=1, 0.4%), and candidal pseudohyphae (n=2, 0.8%). Trophoblastic giant cell, which mimics a malignant cell, is considered normal in pregnancy (Fig. 1). No correlation was noted between Pap smear abnormality and age of coitarche (p=0.99) or duration of marriage (p=0.83).
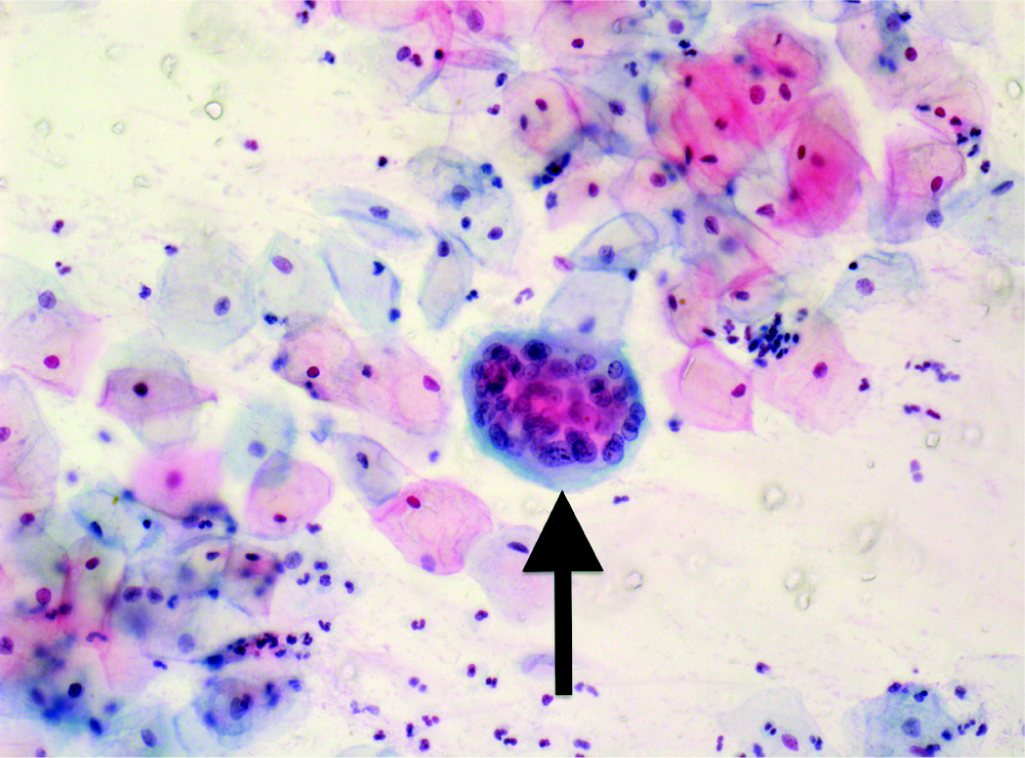
- Trophoblastic giant cell seen under high power (Papanicolaou stain): A physiological variant
Human papillomavirus prevalence
The prevalence of HPV infection in the study population was 8.2% (22/269). The RLUs in HPV-positive individuals ranged from 1.07 to 1816.98, with a median of 3.795. Table I shows the correlation of HPV positivity with various characteristics. HPV prevalence varied from 4.35% to 9.36% in different age groups (p=0.13). It was not found to have a significant association with age at coitarche (p=0.82), duration of sexual activity (p=0.55) or parity (p=0.1).
| Parameter | Human papillomavirus DNA | Total (n=269), n(%) | |
|---|---|---|---|
| Positive (n=22), n(%) | Negative (n=247), n(%) | ||
| Age in years (%) | |||
| 25–29 | 16 (9.4) | 155 (90.6) | 171 (63.6) |
| 30–34 | 3 (4.3) | 66 (95.7) | 69 (25.7) |
| 35–39 | 2 (7.4) | 25 (92.6) | 27 (10.0) |
| 40–44 | 1 (50.0) | 1 (50.0) | 2 (0.7) |
| Age at coitarche (years) | |||
| <18 | 1 (4.2) | 23 (95.8) | 24 (8.9) |
| 18–24 | 14 (14.0) | 158 (86.0) | 172 (63.9) |
| 25–29 | 6 (10.3) | 52 (89.7) | 58 (21.6) |
| >30 | 1 (6.7) | 14 (93.3) | 15 (5.6) |
| Duration of sexual activity (years) | |||
| <5 | 14 (10.1) | 124 (89.9) | 138 (51.3) |
| 6–10 | 7 (6.5) | 101 (93.5) | 108 (40.2) |
| >10 | 1 (4.4) | 22 (95.7) | 23 (8.6) |
| Gravidity | |||
| Primigravida | 4 (7.14) | 52 (92.86) | 56 (20.82) |
| Second gravida | 14 (13.33) | 91 (86.67) | 105 (39.03) |
| Third gravida) | 2 (3.92 | 49 (96.08) | 51 (18.96) |
| Fourth gravida | 2 (3.51) | 55 (96.49) | 57 (21.19) |
| Pap smear | |||
| Normal | 21 (95.5) | 244 (98.8) | 265 (98.51) |
| Abnormal | 1 (4.5) | 3 (1.2) | 4 (1.49) |
The prevalence of abnormal Pap smear (>ASCUS) in HPV-positive women was 4.5% (1/22). Only 1 of 4 women with abnormal Pap smear was HPV-positive. Thus, there was poor correlation between abnormal Pap smear and HPV infection (p=0.29). None of the 3 HPV-vaccinated women were positive on Pap or HPV testing.
Colposcopy
The colposcopy referral rate based on abnormal Pap smear was 1.4% (4/269) and based on abnormal HPV test it was 8.2% (22/ 269). Among 25 subjects referred for colposcopy, only 15 (60%) underwent colposcopic examination (refused 2; long distance residence 3; lost to follow-up 3; placenta previa 1; active candidal infection 1).
Table II shows the correlation between cytology, HPV test, colposcopy (Swede score) and histopathology. The Swede score was <5 in all, i.e. below the risk threshold for determining high-grade lesions (CIN2+). All 15 patients had a type 1 transformation zone with adequate colposcopy. All subjects with an abnormal Pap smear had a Swede score of 0–2. Biopsies were taken for 3 subjects with Swede scores >2: 1 during the antenatal period and 2 during postnatal follow-up. One of the cases reported as ASCUS on Pap smear, was HPV-negative with the Swede score 2, had CIN 1 on biopsy and was advised follow-up.
| S.No. | Pap smear | HPV DNA (RLU) |
Swede score | Histopathology report |
|---|---|---|---|---|
| 1 | ASCUS | 8.315 | 4 | Chronic cervicitis |
| 2 | NILM | 2.13 | 0 | – |
| 3 | NILM | 2.15 | 0 | – |
| 4 | ASCUS | 0.21 | 2 | CIN 1 |
| 5 | NILM | 4.44 | 2 | Chronic cervicitis |
| 6 | NILM | 298.23 | 1 | – |
| 7 | NILM | 20.67 | 0 | – |
| 8 | NILM | 669.26 | 0 | – |
| 9 | NILM | 1.78 | 0 | – |
| 10 | NILM | 10.18 | 0 | – |
| 11 | NILM | 1.28 | 0 | – |
| 12 | NILM | 1.07 | 1 | – |
| 13 | NILM | 1816 | 1 | – |
| 14 | LSIL | 0.77 | 1 | – |
| 15 | NILM | 1.11 | 0 | – |
HPV human papillomavirus RLU relative light units ASCUS atypical squamous cells of undetermined significance NILM negative for intraepithelial lesion or malignancy LSIL low-grade squamous intraepithelial lesion CIN cervical intraepithelial neoplasia
Knowledge, attitude and practice of cervical cancer prevention
Pre- and post-test questionnaires analysed the level of knowledge about cervical cancer, screening tests and willingness to undergo screening during pregnancy and in the future. They also assessed difficulties encountered by the patient and doctor during the conduct of the test as well as their experiences.
Among the 269 subjects enrolled, only 98 (36.4%) had heard about carcinoma cervix. Awareness improved with education: among 32 illiterate women, only 1 (3.1%) was aware of carcinoma cervix, which improved to 76/105 (72.4%) among graduates (p<0.001). Only 6/98 (6.1%) were aware about Pap smear and its role in screening of whom 3 (1.1%) had previously undergone a Pap test, which was reportedly normal. None of them had undergone HPV testing previously. The majority (n=138, 51.3%) were readily willing for the test, 128 (47.6%) were willing after motivation and 3 (1.1%) could be motivated with difficulty. Only 3 patients refused testing, with fear of injury and pain persisting despite motivation.
Pre- and post-test apprehension of subjects
A high proportion of women (n=183; 68.0%) were apprehensive about the test. The majority (n=161, 59.9%) were concerned that the test might be painful, a few had fear of injury (n=11, 4%), embarrassment (n=8, 3.0%), fear of bleeding (n=1, 0.3%), or fear of injury to the foetus (n=2, 0.7%). On post-test assessment, however, only 65 (35.5%) of them reported pain/discomfort, while 114 (62.3%) realized that their apprehensions turned out to be false (Fig. 2). Only 4 (2.2%) of them expressed reluctance to get this test done in the future.

- Comparison of pre- and post-test apprehensions regarding the test
Difficulty during conduct of the test
The majority of women (n=227, 84.4%) had no difficulty in lying down in dorsal lithotomy position for the test. However, of 77 women who were in the third trimester, 30 (39.0%) perceived it to be significantly more uncomfortable compared to those at <28 weeks of gestation (p<0.001).
From the physician’s perspective, the main difficulty was in visualization of the cervix in 93 (34.6%) subjects during the speculum examination. This was significantly greater when the period of gestation was >20 weeks (74/93, p<0.001). In 38 (14.1%) subjects, excessive vaginal discharge obscured the cervix, which was easily cleared with a saline-soaked swab and adequate samples were obtained.
DISCUSSION
Cervical cancer is recognized as a major public health problem and yet systematic screening in LMICs has remained an elusive target. The WHO’s call for elimination of cervical cancer as a public health problem recommends screening 70% of women with a high precision HPV test at the age of 35 and 45 years.10 The problem with meeting this target is two-fold. In a country such as India with a large population of 1.35 billion, the number of women aged 35–45 years is 185.36 million.14 Screening these women even with one or two rounds of testing is a mammoth task, with health facilities that are already stressed with the burden of other communicable and non-communicable diseases. Moreover, there is little awareness of preventive healthcare services and our study also shows the low awareness regarding cervical cancer screening—only 3 (1.1%) subjects had undergone a Pap test in the past and none had had HPV testing. Parkpinyo et al.15 found that 54% of pregnant women had never undergone any cervical screening in their lifetime.
The antenatal period is often the only time that a woman approaches the health system for preventive care. Various guidelines also suggest that Pap smear should be done during pregnancy if it is due.16 However, previous attempts to use this opportunity for cervical cytology screening had proved futile. In the present study, adequate smears were obtained in 98.5%, comparable to the study by Prabhu et al.17 However, the prevalence of abnormal Pap smear (ASCUS or worse), although somewhat higher (1.5%) than other Indian studies which ranged from 0% to 1.0%,17,18 was still very low and the fact that there was no case of high-grade CIN or cancer detected confirms the lack of utility of cytological screening in pregnancy.
However, our study showed the feasibility of using this opportunity to increase awareness about cervical cancer screening and to offer screening for high-risk HPV infection to women likely to be less compliant due to resource limitations and lost to follow-up. Screening during pregnancy was well accepted. Only 3 women refused screening in our study. Counselling and proper information about the procedure play an important role at all steps of the screening process. While 68% (n=183) women were apprehensive initially, they agreed to participate after counselling, and at post-testing, 62.3% of them (114/183) reported that their apprehensions had been unfounded.
A major concern with HPV testing in women under 30 years of age has been the higher incidence and prevalence of HPV infection in the third decade of life in most reports worldwide,19 which raises concerns about high rates of triage and unnecessary treatments. However, this has not been observed in India where the HPV prevalence remains similar across age groups.20 A similar finding has been reported from other LMICs also, although the reason is not clear.21 The prevalence of HPV infection in the present study was 8.2% (n=22), and similar across age groups (p=0.13). It was no higher than the HPV prevalence reported by our group in non-pregnant women aged up to 25 years,22 or in the meta-analysis on HPV prevalence in women over 30 years with normal cytology worldwide, which found that in India the HPV prevalence rate is relatively low (7.8%).23 The only other study from India on the prevalence of HPV infection among antenatal women recruited subjects from age 16 years onwards.24 While they reported an overall prevalence of 18%, it was markedly higher among young women and the prevalence among women aged >25 years was only 3%. Thus, screening women in the age group of 25–30 years for HPV infection is not likely to add to the burden of triage. Pregnant women aged >30 years can be screened during the antenatal period as suggested by various guidelines. There was no association between age at marriage, duration of marriage and abnormal Pap smear or HPV infection, in our study or in other Indian studies.24,25
There was some difficulty in positioning women after 20 weeks of gestation, to take a cervical sample. The early second trimester is thus an ideal time as endocervical cells are translocated outside the cervix, the transformation zone is visualized easily in >80% of cases, and sampling is easier at this time. The endocervical sampler used in conventional Pap smear extends only till the lower half of the cervix and various studies have shown its safety during pregnancy.26,27 We did not encounter any complication such as bleeding from the congested cervix, abortion, infection or preterm labour.
HPV testing has an additional advantage that vaginal self-sampling has been shown to have good concordance with cervical samples.28,29 In future, self-collection of HPV samples is likely to be the preferred method, which can avoid patient discomfort as well as reduce the workload of healthcare workers. A greater concern may be compliance with and timing of colposcopy. We found that only 68% of women complied with the invitation for colposcopy. This has been widely reported and recognized by various other studies.30,31 The development of portable colposcopes combined with affordable tests and increasing awareness about carcinoma cervix can play an important role in improving this outcome and reduce the lost to follow-up rates.32
Antenatal cervical screening provides an excellent opportunity to improve awareness and increase compliance to screening programmes. Screening women who are aged 30 years and above as recommended in the guidelines will improve coverage of the target population.7 It may be a once-in-a-lifetime opportunity for those who do not have regular access to healthcare facilities. The development of affordable point-of-care tests, which is now receiving support, will be key to widespread implementation of HPV testing.
Conclusions
All antenatal women can be informed about cervical cancer screening programmes, the importance of regular follow-up, as well as improving nutrition and menstrual hygiene practices. Universal HPV testing can be offered to women in the target age group in the early second trimester of pregnancy and offers hope of a solution for LMICs that have poor outreach facilities. The establishment of linkages with the identification system can improve the efficacy of the screening programme and have a major impact on eliminating cervical cancer.
Conflicts of interest
None declared
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of screening on incidence of and mortality from cancer of cervix in England: Evaluation based on routinely collected statistics. BMJ. 1999;318:904-8.
- [CrossRef] [PubMed] [Google Scholar]
- Family planning programme in India: Its impact in rural and urban areas, 1970-1980. Delhi: Mittal Publications; 1987. p. :265.
- [Google Scholar]
- Screening for cervical cancer in pregnancy: Our experience. Indian J Public Health Res Dev Regist. 2013;4:202.
- [CrossRef] [Google Scholar]
- Comparison of cervical cancer screening by visual inspection with acetic acid versus cervical-cytology in pregnancy. Indian J Med Paediatr Oncol. 2019;40:85.
- [CrossRef] [Google Scholar]
- Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:98-108.
- [CrossRef] [PubMed] [Google Scholar]
- Screening and management of preinvasive lesions of the cervix: Good clinical practice recommendations from the Federation of Obstetrics and Gynaecologic Societies of India (FOGSI) J Obstet Gynaecol Res. 2020;46:201-14.
- [CrossRef] [Google Scholar]
- Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2017;3:635-57.
- [CrossRef] [PubMed] [Google Scholar]
- 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17:S1-S27.
- [CrossRef] [PubMed] [Google Scholar]
- Draft: Global Strategy towards Elimination of Cervical Cancer as a Public Health Problem. 2020. Geneva: WHO; Available at www.who.int/publications/m/item/draft-global-strategy-towards-eliminating-cervical-cancer-as-a-public-health-problem (accessed on 8 Nov 2020)
- [Google Scholar]
- Global Delivery Care Coverage and Trends: UNICEF. 2020. Available at https://data.unicef.org/topic/maternal-health/delivery-care/ (accessed on 22 May 2020)
- [Google Scholar]
- The Swede score: Evaluation of a scoring system designed to improve the predictive value of colposcopy. J Low Genit Tract Dis. 2010;14:301-5.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of the strength of association of Reid colposcopic index and Swede score with cervical histology. J Low Genit Tract Dis. 2017;21:55-8.
- [CrossRef] [PubMed] [Google Scholar]
- World Bank Staff Estimates Based on Age/Sex Distributions of United Nations Population Division's World Population Prospects. 2019. Available at https://data.worldbank.org/indicator/SP.POP.3539.FE.5Y?locations=IN (accessed on 28 Mar 2020)
- [Google Scholar]
- Benefits of cervical cancer screening by liquid-based cytology as part of routine antenatal assessment. Asian Pac J Cancer Prev. 2016;17:4457-61.
- [Google Scholar]
- Clinical pregnancy guidelines: Pregnancy care. Canberra:Australian Government Department of Health 2018:320. Available at www.health.gov.au/sites/default/files/pregnancy-care-guidelines_0.pdf (accessed on 4 Nov 2020)
- [Google Scholar]
- Opportunistic cervical cancer screening in pregnancy. Int J Med Res Health Sci. 2016;5:278-81.
- [Google Scholar]
- Cervical cytological changes detected by Papanicolaou smear in antenatal patients attending a tertiary care centre. Eur J Cancer. 2014;50:e13-e14.
- [CrossRef] [Google Scholar]
- Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect Dis. 2007;7:453-9.
- [CrossRef] [PubMed] [Google Scholar]
- Papillomavirus infection in rural women in southern India. Br J Cancer. 2005;92:601-6.
- [CrossRef] [PubMed] [Google Scholar]
- Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677-84.
- [CrossRef] [PubMed] [Google Scholar]
- Type-specific incidence and persistence of HPV infection among young women: A prospective study in North India. Asian Pac J Cancer Prev. 2012;13:1019-24.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789-99.
- [CrossRef] [PubMed] [Google Scholar]
- A study of cervical intraepithelial neoplasia in pregnancy. J Obstet Gynaecol India. 2014;64:193-6.
- [CrossRef] [PubMed] [Google Scholar]
- Expanding horizon of cervical cancer screening by involving antenatal patients. J Med Sci Clin Res. 2016;4:14805-13.
- [CrossRef] [Google Scholar]
- Evaluation of the endocervical Cytobrush and Cervex-Brush in pregnant women. Obstet Gynecol. 1994;84:539-43.
- [Google Scholar]
- The effectiveness and safety of two cervical cytologic techniques during pregnancy. J Fam Pract. 1997;45:159-63.
- [Google Scholar]
- Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009;33:446-50.
- [CrossRef] [PubMed] [Google Scholar]
- Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ. 2018;363:k4823.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for cervical and breast cancer: Department of Health and Family Welfare Government of Tamil Nadu. Available at https://tnhsp.org/tnhsp/screening-cervical-cancer-and-breast-cancer.php (accessed on 5 Nov 2020)
- [Google Scholar]
- The challenge of follow-up in a low-income colposcopy clinic: Characteristics associated with noncompliance in high-risk populations. J Low Genit Tract Dis. 2012;16:345-51.
- [CrossRef] [PubMed] [Google Scholar]
- A crossover randomized study of portable transvaginal colposcope and hand-held colposcope for evaluation of cervical intraepithelial neoplasia. Int J Gynecol Obstet. 2018;143:991.
- [Google Scholar]




