Translate this page into:
Evaluation of satisfaction and reasons for participation in a Covid-19 vaccine clinical trial: A single-centre, observational study
Correspondence to NITHYA J. GOGTAY; njgogtay@hotmail.com
[To cite: Kudyar P, Soni D, Gogtay NJ. Evaluation of satisfaction and reasons for participation in a Covid-19 vaccine clinical trial: A single-centre, observational study. Natl Med J India 2022:35:214–18.]
Abstract
Background
In May 2020, WHO recognized the role of extensive immunization for interrupting the transmission of the SARS-CoV-2 virus. The development of such vaccines in clinical trials relies upon participants who are expected to be vested in the research process. Assessment of participant factors such as motivation and satisfaction are hence important to gauge perspective and ensure successful conduct and completion of these trials.
Methods
We administered a validated three-domain questionnaire to and documented the binary categorical responses (yes/no) of participants (after informed consent) who had taken both doses of COVOVAX™ in a phase 3 trial at our institute. Association of the dependent variables (participant responses) with the independent variables (participant demographics and socioeconomic strata) was computed using Chi-square test at 5% significance. In case of a significant association, Bonferroni post-hoc test was applied for multiple comparisons.
Results
Of the 78 participants who were administered the questionnaire, two-thirds were highly satisfied with their experience at our site. Gaining access to a new vaccine was a primary motivation overall (74%) and also in graduates (p=0.03) and middle-class population (p=0.002), whereas the lower-middle class population (p<0.0001) and those educated till secondary school (p=0.003) took part due to the long wait for government-approved vaccines.
Conclusion
Participants in a Covid-19 vaccine trial at Mumbai were largely satisfied with the care given to them though altruism did not feature as a primary reason for participation.
INTRODUCTION
The development of a safe and effective vaccine has been a challenge for scientists since the start of the Covid-19 pandemic.1 Till 3 June 2022, a total of 38 Covid-19 vaccines have got approval at least in one country while 205 vaccine candidates are available worldwide for which 715 clinical trials are ongoing.2 Clinical trials have been the mainstay of vaccine development in this ongoing pandemic. The conduct of successful, high-quality trials with rapidity in a pandemic relies on enrolling and retaining healthy participants who are vested in and understand and trust the research process.3 Their motivation and satisfaction is a key factor in the successful completion of these studies.
There could be several reasons as to why otherwise healthy people choose to participate in clinical trials, especially in a pandemic. An assessment of these factors will give us an insight into the reasons and help plan future trials..4 We aimed to assess the satisfaction and factors that motivated participants to take part in a Covid-19 vaccine trial conducted at a tertiary referral hospital that houses a clinical research department.
METHODS
Ethics
The study was approved by the institutional ethics committee (EC-OA/184/2021). As per the directive of the institutional ethics committee, participants’ consent was obtained verbally and this was audio recorded over the telephone. The trial was registered prospectively with the Clinical Trials Registry of India (CTRI/2022/03/040969).
Eligibility criteria
Participants who had consented for a Covid-19 vaccine trial at our centre (CTRI/2021/02/031554, two-dose vaccine) formed the study sample. All participants who provided consent were included in the study. Those who withdrew consent and those who did not complete the study (a total of 6 study visits) for any reason were also included. Those who had not taken both doses of the vaccine were excluded.
Questionnaire development
We developed our own questionnaire for the study. It consisted of 15 questions divided into three domains with binary (yes/no) responses. This questionnaire was based on the personal experiences with any vaccine trial in the past by the senior author and the general reasons enunciated by participants in these studies. The domains were: (i) satisfaction from participation in the study; (ii) factors that motivated them to participate; and (iii) motivation for participation in future vaccine trials. The satisfaction domain had 8 questions with yes or no responses. The minimum possible score was 0 and maximum 8. The domain that assessed factors that motivated the participant to volunteer for the Covid vaccine trial had 5 factors and more than one response could be checked. The final domain had 2 questions with binary responses (Table I).
| Domain 1: Participant satisfaction |
| 1 . Facility was conducive for the study |
| 2 . Research staff was knowledgeable and explained the procedures |
| 3 . Was fully informed of risks and benefits for participation |
| 4 . Understood my participation is voluntary and can withdraw at any time |
| 5 . Research staff was approachable for my questions and concerns |
| 6 . Sufficient time was spent with me |
| 7 . Received reminders about upcoming visits |
| 8. Overall experience was positive |
| Domain 2: Motivation factors for participation |
| 1 . To gain access to the new Covid vaccine |
| 2 . To gain access to the Covid vaccine due to the long wait for government-approved vaccines |
| 3. To help society (altruism) |
| 4 . For financial gains |
| 5 . To get a free routine health check-up done (RTPCR and Covid antibody check-up) which otherwise would cost me money |
| Domain 3: Motivation for future participation |
| 1 . Would like to participate in any further research at the trial centre |
| 2 . Would recommend others to participate in such research studies |
Questionnaire validation
This was done using the Kuder–Richardson 21 formula5 which is a specialized form of Cronbach alpha measure for reliability of a test with binary variables. The scores for KR-21 range from 0 to 1, where 0 is no reliability and 1 is perfect reliability. The closer the score is to 1, the more reliable the test. A questionnaire score of over 0.5 is usually considered reasonable. The questionnaire was administered to 30 people whose replies were then transcribed in an Excel sheet and found to have a score of 0.66.
Questionnaire administration
Initially, we contacted the participants telephonically. Subsequently, the study was explained in detail to them in the language comfortable to them. They were given the choice of declining consent. After taking and recording their verbal consent, questions from all the domains in the questionnaire were read out to the participants slowly one by one and their replies were noted.
Outcome measures
We recorded identifiers such as age and gender, education and employment status. Using the per capita family income of the participants, they were classified into various socioeconomic classes using the BG Prasad scale6,7 (Table II). The BG Prasad scale, first introduced in 1961 is the one of the most widely used scales to classify the socioeconomic status based on the family’s per capita income.8 Over the years, it has been modified to incorporate the dynamic effect of the consumer price index on the per capita income leading us to use this scale for assessment of social class. Other outcome measures included (i) proportion of participant satisfaction scores after volunteering; (ii) proportion of participant responses to specific factors that motivated them to participate; (iii) proportion of participant responses to saying yes to future studies; and (iv) association of dependent variables (satisfaction, motivation and future motivation) with the independent variables (age, gender, employment, education status, socioeconomic status).
| Socioeconomic class | Per capita income/month (₹) | |
|---|---|---|
| Class I | Upper class | 8224 and above |
| Class II | Upper-middle class | 4112–8223 |
| Class III | Middle class | 4111–2467 |
| Class IV | Lower-middle class | 1234–2466 |
| Class V | Lower class | 1233 and below |
Statistical analysis plan (SAP)
Both descriptive and inferential statistics were applied to the data. Categorical data such as age, gender, education, employment status and socioeconomic class were expressed as proportions. Participant responses for each factor (satisfaction score, motivation reason, future motivation) were also expressed as proportions. Association of the dependent variables (participant responses) with the independent variables (demographics and socioeconomic strata) was computed using Chi-square test. If and when a significant association was found in Chi-square test, Bonferroni post-hoc test was applied for multiple comparisons. All statistical tests were done using SPSS, Version 25 (IBM Corp., Armonk, New York, USA, 2017) at 5% significance.
RESULTS
Demographics
A total of 97 participants had completed both doses of the vaccine and were contacted telephonically. Of these, 78 (80%) consented to participate in the study. There were 72 (92%) men and 6 (8%) women. Of these, 2 participants had completed only two visits of the study. Forty-six per cent of the participants belonged to the age group of 18–35 years. Eighty-three per cent were either graduates or were educated till secondary school; 68% of them were also employed (Tabe III).
BG Prasad scale
A total of 15/78 (19%) and 26/78 (33%) of the participants in the study were classified as upper class and upper-middle class, respectively, 25/78 (32%) were classified as middle class whereas 7/78 (9%) were lower-middle class and 5/78 (6%) lower class. The details of the demographic profile of the participants are given in Table III.
| Variable (n=78) | n(%) | |
|---|---|---|
| Age group | 18–35 years (young adults) | 36 (46) |
| 36–55 years (middle age) | 33 (42) | |
| 56 years and older (older) | 9 (12) | |
| Gender | Men | 72 (92) |
| Women | 6 (8) | |
| Education | Primary school (till class 8) | 13 (17) |
| Secondary school (till class 12) | 34 (43) | |
| Graduate and above | 31 (40) | |
| Employment status | Employed | 53 (68) |
| Unemployed | 25 (32) | |
| Socioeconomic class | Class I (upper class) | 15 (19) |
| Class II (upper-middle class) | 26 (33) | |
| Class III (middle class) | 25 (32) | |
| Class IV (lower-middle class) | 7 (9) | |
| Class V (lower class) | 5 (6) |
Participant responses to the questionnaire
Domain 1—Satisfaction. A total of 47/78 (60%) participants had a 100% satisfaction score of 8/8, while 21/78 (27%) of them had a score of 7/8 and 10/78 (13%) had a score of 6/8. No participant scored less than 6 on the questionnaire.
Domain 2—Motivation for participation. We found that gaining access to a new Covid vaccine (74%) was the primary reason for participation in the trial, while 31/78 (40%) took part to get a free health check-up done. Approximately one-third 29/ 78 (37%) took part in the study as the vaccine was free and for their age group and payment in the the private sector was not possible for them (at the time of this study, vaccines were not provided free by the government for this age group). Another one-third 28/78 (36%) cited altruism as their reason for participation. A quarter, 21/78 (27%) had participated to avail travel reimbursements given as part of the study.
Domain 3—Motivation for future participation. Almost all the participants 76/78 (97%) were motivated to take part in any further research at the trial site and 69/78 (89%) were also ready to recommend others for such trials.
Impact of participant variables on their responses
We did not find any association of the independent variables of age group, gender and employment status with any of the dependent variables (p>0.05).
Educational level
The satisfaction score for the trial site experience was significantly higher among those who were either graduates or had a degree higher than graduation (84% had a score of 8/8) (p<0.001) while those educated until primary school or lower had much lower satisfaction scores (62% had a score of 6/8) (p<0.00001).
We found that graduates and those with higher than a graduate degree were significantly more motivated to participate in the trial to gain access to a new Covid vaccine compared to those with lower education (p=0.03). Similarly, graduates were altruistic compared to the rest though the association did not reach statistical significance. Those who were educated till secondary school had a significantly higher motivation for taking the trial vaccine due to the long wait for government-approved vaccines compared to those who were graduates or above (p=0.003).
Participants with education till primary school cited a significantly greater motivation to participate in any future trials than the others (p<0.001).
Socioeconomic status
The participants categorized as upper class gave a significantly greater satisfaction score to their trial experience as per their responses to the questionnaire compared to the other socioeconomic classes (p=0.004) (Fig. 1).
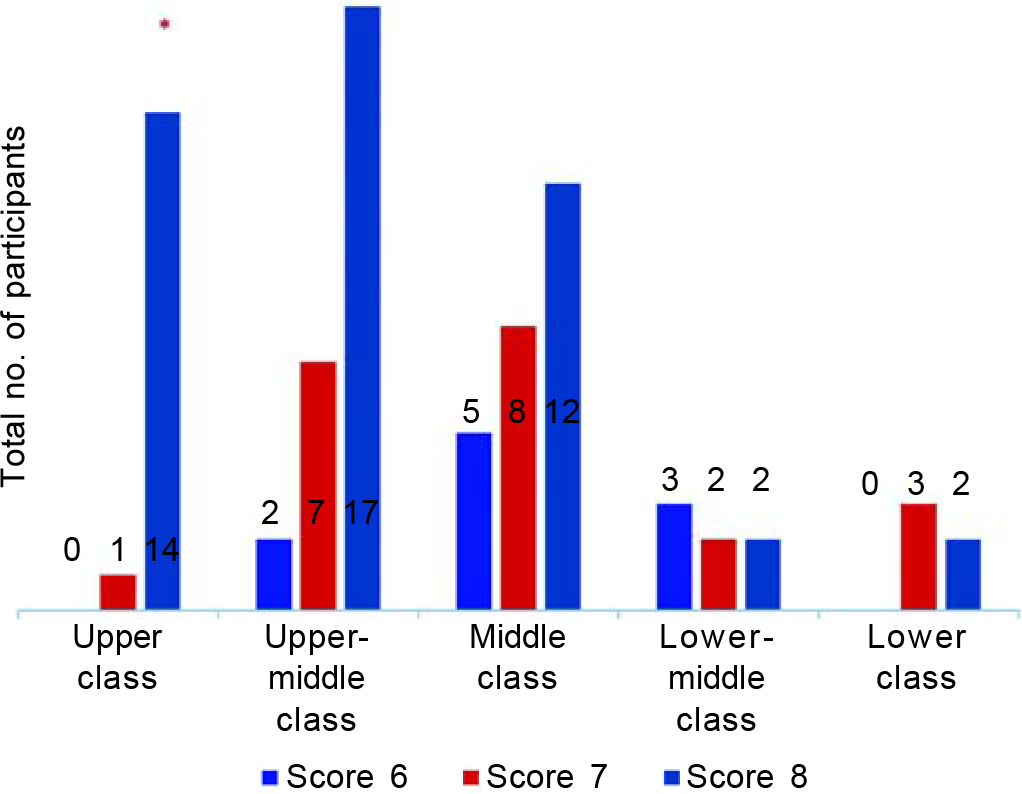
- Satisfaction scores of various socioeconomic groups
The middle-class population was significantly more motivated to participate in the trial to gain access to a newer vaccine for Covid (p=0.002) while the participants classified under the lower-middle class category had a significantly higher motivation for taking the trial vaccine due to the long wait for government-approved vaccines (p<0.0001). Though all the participants under the lower-class category were motivated to participate in the vaccine trial to get a health check-up done compared to the others, but the association was not found to be statistically significant (p>0.05).
DISCUSSION
We assessed satisfaction and reasons for motivation in 78 healthy participants (92% were men) who took part in a Covid-19 vaccine trial at our institute. We found that two-thirds of participants were satisfied with their treatment and care given to them as part of the trial. Gaining access to a Covid-19 vaccine, free medical check-ups were the primary reasons for participation followed by altruism. Graduates and those from the upper socioeconomic strata had better satisfaction scores compared to non-graduates and those from other socioeconomic strata.
From the original clinical trial, only those who consented to participate in the present study were included and the proportion of men was high at 92%. This was also seen in the regulatory trial where 92% of the participants were men. As a negative pregnancy test was a selection criteria in the original trial along with the use of contraception till the 36-day visit, this may have led to the exclusion of women participants. This also reflects that women as a group have been historically largely unrepresented in clinical trials.9 Over the years, with regulatory backing, there has been an increase in representation of women in clinical trials.10 Steinberg et al. evaluated not just the representation but also representation relative to the disease burden in a developed country and found women to be underrepresented in paediatric and oncology trials.11 As Covid-19 is a disease that cuts across all ages, this under-representation in a metropolitan city like Mumbai is worrisome. Potential reasons could be lack of autonomy as shown by us in an earlier study by Figer et al., and the worry about risk to the foetus precluding their participation.12 Abdelhafiz et al., in their questionnaire-based study on 576 healthy participants in Egypt, also found that women were more likely not to take part in Covid-19 trials compared to men.13
The regulatory trial was done for a new Covid-19 vaccine and most healthy persons who consented to participate were either in age range for young adults (18–35 years) or middle age (36– 55 years). We can only surmise about the reasons for participation of very few older participants. These could be frailty, comorbidities, need for travel to the hospital for follow-up, which would have required a caregiver and therefore posed challenges regarding compliance with the trial protocol.14
A total of 13% of participants were not entirely satisfied with the trial. The major reason of dissatisfaction was that sufficient time was not spent with them by the trial team. This may have been due to the stringent timelines of recruitment at our site and a small trial team. Bassi et al. made a similar observation that research studies in India are challenging due to reasons such as limited workforce, and scarce facilities leading to operational difficulties.15 During Covid-19, this would have been magnified due to the pressure on the workforce that was not just working for the trial but was also involved in care for Covid-19 patients. More time may be given to individual participants in future studies as a greater investment in time leads to a better understanding in research participants.16
Almost three-fourths of the study population stated that they were motivated to participate in the trial to gain access to a new Covid vaccine. The Covid-19 pandemic has put tremendous pressure on researchers, regulators as well as policy-makers to bring new vaccines into the market to make it available for the general public in a timely manner.17 At the time this trial was done, the vaccines available in India were Oxford-Astra Zeneca vaccine locally referred to as Covishield™, Bharat BiotechICMR (Indian Council of Medical Research) indigenous vaccine named Covaxin™ and the imported Russian Sputnik V™ vaccine.18 Despite this, there was hesitancy in certain sections of the population towards these vaccines and there was a quest for newer remedies. In a study by Danabal et al. on 596 persons in southern India, the primary reason for vaccine hesitancy was lack of sufficient credible information.19 In contrast to our results, in northern India, monetary gains were found to be a major reason for participation.20 It is difficult to assess the reason for motivation; we can only hypothesize about it. Contracting serious/severe disease is another possibility for which the participants wanted access to the vaccine.
We found that those with higher education were more satisfied. Other studies have also shown that participants with higher education understand research better.21–24 Understanding the trial process is essentially via the informed consent form. The lengthier the form, the more tenuous it is for the participant who is less educated. Grant et al., in their review, stated that the longer the informed consent document is, the less likely it is for people to read and understand it fully.25 The consent form in our clinical trial was nine pages long which could have been a deterrent for those who were less educated.
The vaccine rollout in India began from 16 January 2021, initially only in the public sector for the healthcare workers and for those above 60 years of age, whereas all individuals above 18 years of age were made eligible to receive them only from 1 May 2021,26 leading to a long wait for the vaccines to be made available from the government sector. This group was likely to have said yes to the vaccination trial to gain access and not being able to pay for the vaccine in the private sector.
Our study is restricted to a largely male population at a single centre and of a single Covid-19 vaccine trial and needs to be viewed in that perspective. Also, the questionnaire developed by us may not have captured all facets of satisfaction and motivation. The use of telephone as a tool for communication was an inherent limitation as it was impersonal without direct contact. This could have impacted responses given by the participants and also the lack of control of the investigator over the environment of the participant with potential distracters.27
In summary, 78 participants in a Covid-19 vaccine trial at Mumbai were largely satisfied with the care given to them though altruism did not feature as a primary reason for participation.
Conflicts of interest
None declared
References
- The COVID-19 vaccination acceptance/hesitancy rate and its determinants among healthcare workers of 91 countries: A multicenter cross-sectional study. EXCLI J. 2022;21:93-103.
- [Google Scholar]
- Covid 19 Vaccine tracker. Available at https://covid19.trackvaccines.org/ (accessed on 4 Jun 2022)
- [Google Scholar]
- Satisfaction and perceptions of research participants in clinical and translational studies: An urban multi-institution with CTSA. J Clin Transl Sci. 2020;4:317-22.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of a research participant satisfaction survey at an academic medical center. Clin Res (Alex). 2016;30:42-7.
- [Google Scholar]
- Kuder Richardson Score. Available at https://www.statisticshowto.com/kuder-richardson/ (accessed on 19 Jan 2022)
- [Google Scholar]
- Updated BG Prasad Socioeconomic status classification for the year 2021. J Indian Assoc Public Health Dent. 2021;19:154-5.
- [CrossRef] [Google Scholar]
- BG Prasad Scale. Available at http://labourbureau.gov.in/ (accessed on 26 Feb 2022)
- [Google Scholar]
- Modified BG Prasad Socio-economic Classification updated-2020. Indian J Comm Health. 2020;32:124-5.
- [CrossRef] [Google Scholar]
- Sex and gender factors in medical studies: Implications for health and clinical practice. JAMA. 2003;289:397-400.
- [CrossRef] [PubMed] [Google Scholar]
- Progress and opportunities for women in clinical trials: A look at recent data and initiatives from the US FDA. Med. 2021;2:456-504.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of female enrolment and participant sex by burden of disease in US clinical trials between 2000 and 2020. JAMA Netw Open. 2021;4:2113749.
- [CrossRef] [PubMed] [Google Scholar]
- A survey of knowledge and variables influencing perceptions about clinical research: A cross-sectional study from Mumbai. Perspect Clin Res. 2021;12:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- Factors influencing participation in COVID-19 clinical trials: A multi-national study. Front Med (Lausanne). 2021;8:608959.
- [CrossRef] [PubMed] [Google Scholar]
- Elderly patients' participation in clinical trials. Perspect Clin Res. 2015;6:184-9.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in operationalising clinical trials in India during the COVID-19 pandemic. Lancet Glob Health. 2022;10:e317-e319.
- [CrossRef] [PubMed] [Google Scholar]
- Interventions to improve research participants understanding in informed consent for research: A systemic review. JAMA. 2004;292:1593-601.
- [CrossRef] [PubMed] [Google Scholar]
- How COVID-19 has fundamentally changed clinical research in global health. Lancet Glob Health. 2021;9:e711-e720.
- [CrossRef] [PubMed] [Google Scholar]
- Strategy for COVID-19 vaccination in India: The country with the second highest population and number of cases. NPJ Vaccines. 2021;6:60.
- [CrossRef] [PubMed] [Google Scholar]
- Attitude towards COVID 19 vaccines and vaccine hesitancy in urban and rural communities in Tamil Nadu, India: A community based survey. BMC Health Serv Res. 2021;21:994.
- [CrossRef] [PubMed] [Google Scholar]
- Factors influencing participation of healthy volunteers in clinical trials: Findings from a cross-sectional study in Delhi, North India. Patient Prefer Adherence. 2019;13:2007-15.
- [CrossRef] [PubMed] [Google Scholar]
- Information and communication in the context of a clinical trial. Eur J Cancer. 2000;36:2096-104.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial assessing the impact of written information on outpatients' knowledge about and attitude toward randomized clinical trials. The INFO trial group. Control Clin Trials. 2000;21:223-40.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized clinical trials in oncology: Understanding and attitudes predict willingness to participate. J Clin Oncol. 2001;19:3554-61.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of informed consent in cancer clinical trials: A cross-sectional survey. Lancet. 2001;358:1772-77.
- [CrossRef] [PubMed] [Google Scholar]
- Informed consent-We can and should do better. JAMA Netw Open. 2021;4:2110848.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges facing COVID-19 vaccination in India: Lessons from the initial vaccine rollout. J Glob Health. 2021;11:3083.
- [Google Scholar]
- Is there a bias against telephone interviews in qualitative research? Res Nurs Health. 2008;31:391-8.
- [CrossRef] [PubMed] [Google Scholar]




