Translate this page into:
Finger printing of counterfeit bevacizumab and identifying it before clinical use
Corresponding Author:
Thirumurthy Velpandian
Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
tvelpandian@hotmail.com
| How to cite this article: Velpandian T, Nath M, Laxmi M, Halder N. Finger printing of counterfeit bevacizumab and identifying it before clinical use. Natl Med J India 2016;29:326-329 |
Abstract
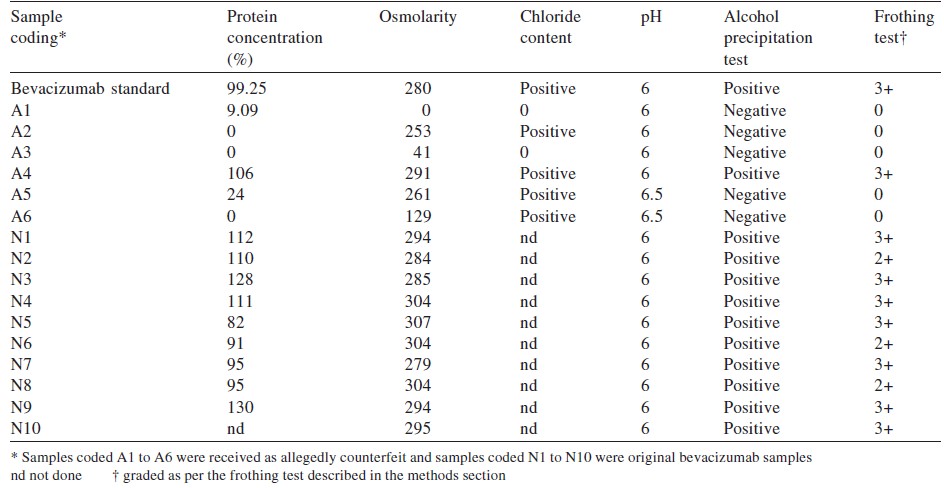
Background. Bevacizumab is widely used for ophthalmic purposes. Recently, counterfeit bevacizumab has become a matter of concern. We analysed samples of suspected counterfeit formulations of bevacizumab and assessed the possibility of using simple tests in the clinic by ophthalmologists to prevent the use of counterfeit preparations in patients.Methods.We did a protein analysis using Bradford assay and SDS-PAGE to confirm the presence of bevacizumab in 16 samples - 6 suspected and 10 others. The samples were also subjected to physicochemical analysis such as osmolarity, chloride content and pH. The samples tested negative for protein were analysed by mass spectrometry to detect drugs used in place of bevacizumab. We standardized the method of frothing and precipitation analysis for identifying authentic samples of bevacizumab before their clinical use.
Results. Five of the 16 samples tested were negative for the presence of bevacizumab. The physicochemical parameters also supported the protein analysis test. However, no ionizable organic compound (other drug[s]) was detected by mass spectrometry.
Conclusion. Ophthalmic use of counterfeit bevacizumab can be prevented by simple methods such as the frothing and precipitation tests. These can identify the absence of an active drug.
Introduction
Bevacizumab is a recombinant, humanized, monoclonal antibody against vascular endothelial growth factor (VEGF). It is designed to bind to and inhibit VEGF, which plays an important role in tumour angiogenesis--a process critical for tumour growth and metastasis.
Although it has been approved by the US Food and Drugs Administration (FDA) for metastatic colorectal cancer, off-label intravitreal use of bevacizumab is prevalent among ophthalmologists worldwide. Bevacizumab has been found to be well tolerated and is devoid of any significant retinal toxicity in various preclinical and clinical studies. Therefore, cost-effective formulations of bevacizumab can be explored as an alternative and economical approach to treat a variety of retinal pathologies in resource-poorsettings. [1] For the past 9 years, we have successfully dispensed more than 600 vials of bevacizumab in sterilized glass ampoules for intravitreal injection. These vials are regularly subjected to quality control checks for sterility.
Quality control measures to detect counterfeit bevacizumab have been made more stringent in the USA and Canada by including protein content analysis after warnings by the US FDA and Genentech. After detecting one such vial with no protein, the search was widened at our centre from October 2014 to collect suspected bevacizumab vials from various parts of India. We evaluated the protein content in suspected counterfeit vials, and assessed the possibility of using simple test(s) to identify such samples in the clinic before use in patients.
Methods
Collection of samples
Sixteen samples (13 unopened vials, 2 opened vials and 1 small amount from an opened vial) were analysed. We received suspected samples from Delhi, Kolkata and Hyderabad. Of the 16 samples, 6 were suspected counterfeit samples and the remaining 10 unopened vials were taken from the stock of Dr Rajendra Prasad Centre for Ophthalmic Sciences, New Delhi.
Analysis for IgG using SDS polyacrylamide gel electrophoresis
Protein molecular weight of the samples was determined by SDS-polyacrylamide gel electrophoresis. Bevacizumab samples were prepared in reducing sample buffer and heated to 90 °C for 10 minutes before loading on 10% SDS-polyacrylamide gel using a Tris/Glycine buffer. For standard 1 μg and for each sample 10 μg was loaded and gels were run at 50 mA. Gels were stained with Coomassie Blue R-250 (Biorad, USA) to see the protein band.
Quantification of protein using Bradford
Bradford reagent was prepared by dissolving 50 mg of Coomassie brilliant blue G-250 (SD Fine, Mumbai, India) in 50 ml of ethanol (Merck, USA) and adding 100 ml 85% (w/v) phosphoric acid (H 3 PO 4 ) (SD Fine, Mumbai, India). This acid solution was slowly mixed with 850 ml of water, filtered and kept at 4 °C till further use. Standards of bovine albumin were prepared at five different concentrations of 2, 4, 8, 16 and 20 μg/ml using working solution of distilled water and 10 μl of different bevacizumab samples; and standards were incubated with 300 μl of Bradford reagent for 5 minutes at room temperature. The reaction was stopped using 10 μl of 0.1 M hydrochloric acid. The mixture was vortexed and 200 μl of the prepared solution was loaded in a 96 well plate and read at 595 nm using Spectramax Paradigm, Multimode detection platform (Molecular Devices, Austria).
Quick protein analysis using UV spectrophotometer
Samples were appropriately diluted to obtain expected concentrations of 10 μg/ml based on the label claim of 25 mg/ml, in distilled water and were then subjected to ultraviolet (UV) spectroscopy (200-400 nm) using UV1 (Thermo Spectronic, England, UK) spectrophotometer against distilled water as a blank. Albumin (SD Fine, Mumbai, India) was used as a positive control and distilled water as a negative control.
Osmolarity analysis
All the samples were analysed for osmolarity using μ-Osmette (Precision System, USA), which was calibrated using standard sodium chloride as per the manufacturer′s instructions. Normal saline was used as a positive control and distilled water as a negative control.
Chloride content analysis in suspected counterfeit formulations
Chloride content was tested in those samples which did not detect any protein by the Bradford or by UV assay. Briefly, the samples were added to 1 ml solution of silver nitrite and nitric acid. To confirm the presence of chloride, standard saline was used as a positive control and distilled water as a negative control. Immediate formation of white precipitate was considered positive for the presence of chloride.
Frothing test and alcohol precipitation method
All samples found positive and negative for protein and antibody were coded and subjected to the following tests. The personnel who performed the test were unaware of the contents of the vials. A sample of 25 mg/ml egg albumin (SD Fine, USA) was used as a positive control and distilled water as a negative control.
Frothing test. The vial was shaken vigorously for 15 seconds and then kept undisturbed for one minute. After one minute, the vial was examined from the bottom using a torch light. Frothing was graded as:
0 no frothing on the sides
1+ slight frothing on the sides
2+ stable frothing on the sides
3+ stable frothing on the sides as well as in the centre.
Alcohol precipitation test. For the ease of use of this test in clinics, commercially available 75% alcohol (Sterilium, Raman & Weil, Mumbai, India) containing a mixture of 2-propanol (45% w/w), 1 propanol (30% w/w) and ethyl-hexadecyl-dimethyl-ammonium ethyl sulphate (0.2% w/s) was used.
In a clean curved spatula, 100 μl of the above 75% alcohol-based sterilizing solution was taken, and to this one drop of bevacizumab solution was added using a syringe. A quick formation of white precipitate was considered positive for the presence of protein. This test was conducted independently by two persons who were unaware of the contents of the syringe, the individual observations and of the results of the chemical analysis. Saline was used as a negative control.
Information-dependent acquisition (IDA) protocol analysis of suspected samples. The samples found negative for proteins by the above method were analysed using ESI-LC-MS/MS. Chromatographic separation was achieved using C18 (Purospher star, RP-18 endcapped, 3 μm, Darmstadt, Germany). The liner gradient mobile phase reaching 100% acetonitrile (with 0.1% formic acid) from 5% over 8 minutes was used to elute the compounds. The mobile phase was used at the rate of 0.5 ml/minute. Source parameters such as nitrogen gas 1 and 2 were kept at 30 and 60 psi, respectively at the ESI temperature of 450 °C. The IDA protocol consisting of enhanced mass spectrometry (EMS), enhanced resolution (ER) and enhanced production scan (EPI) was performed at the speed of 4000 Da/second between 100 and 800 a.m.u. at the positive ESI (+5500 V) temperature of 450 °C. After acquisition of data, EMS scan was used for UV photodiode array spectrum-guided analysis of suspected samples.
Results
We received suspected samples from Susrut Eye Hospital and Research Foundation, Kolkata; Neo Retina Eye Care Institute, Hyderabad; and Disha Eye Hospital, Kolkata (original vial sent to Roche, Switzerland by the hospital for analysis). No apparent difference was observed in the labelling of the counterfeit and original vials of bevacizumab.
Analysis of vials for its contents
The Bradford test was negative for detectable protein in 5 samples ([Table - 1]). SDS polyacrylamide gel electrophoresis showed the absence of light and heavy chain bands representing IgG in the 5 samples tested negative for protein ([Figure - 1]).
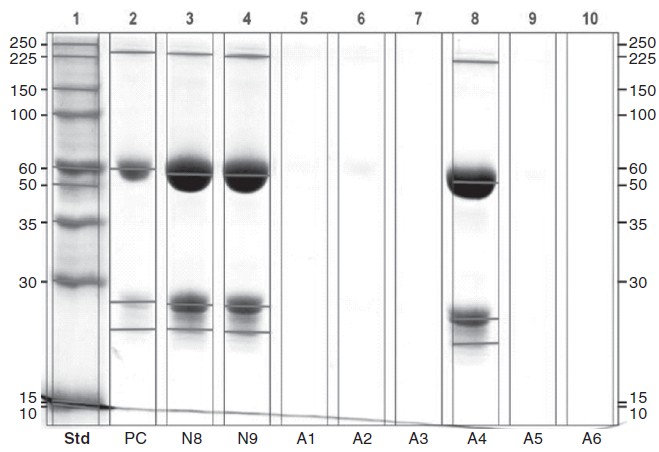 |
| Figure 1: SDS PAGE blot with bevacizumab standard (lane 2), absence of bevacizumab in counterfeit vials (lanes 5 to 7, 9 and 10) and containing bevacizumab (lanes 3, 4 and 8). PC positive control |
UV spectrophotometric analysis
UV spectrophotometric analysis showed absence of any other interfering compound having UV absorption in the samples tested negative for proteins. The samples that were tested positive for the presence of IgG and protein showed a peak at 280 nm ([Figure - 2]).
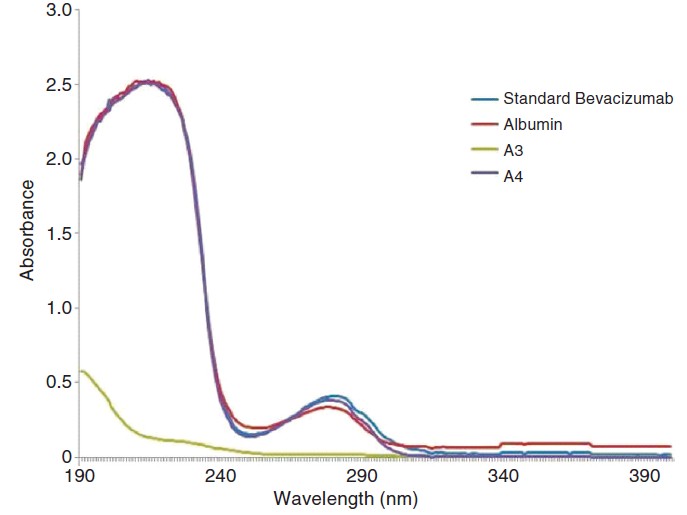 |
| Figure 2. Ultraviolet spectra of the bevacizumab positive (A4, albumin and bevacizumab standard) and counterfeit vial (A3) |
Frothing test and alcohol precipitation method
Five of 16 samples were tested negative by the frothing and precipitation methods. Other positive samples were marked in the range 2+ to 3+. All frothing samples showed precipitation in the alcohol test ([Table - 1]).
Analysis of samples by mass spectroscopy for the presence of organic compounds
The IDA analysis did not show any ionizable organic compound in detectable amounts for further identification.
Discussion
Counterfeit drugs, which pose a challenge to health of patients, are increasingly being reported in the USA but their exact prevalence there or elsewhere is unknown. Improved drug surveillance has been advocated after the warning by the US FDA about counterfeit vials of bevacizumab, and identification of populations at risk to such medications. [6]
Although internet pharmacies have been suspected to be a source for introducing counterfeit drugs in the USA [7] and Japanese markets, [8] the profits accrued by dispensing counterfeit formulations are lucrative enough for their penetration in the regular wholesale/retail supply chain in developing countries such as India where internet pharmacies have a small presence.
In the usa, 0 bevacizumab is marketed by Genentech (a member of Roche Group). In India, it is imported and marketed by Roche Products, India. The product used in India is manufactured by Roche Diagnostics, GmbH, Germany for F. Hoffman-La Roche, Basel, Switzerland. The product for India comes in a packaging of 100 mg in 4 ml, which is different from that available in the US market.
In view of its safety and efficay, bevacizumab has been used intravitreally in ocular neovascular complications including retinopathy, macular oedema due to diabetic complications and chorodial neovascularization. This off-label use of less-expensive bevacizumab is preferred for those who are not covered by medical insurance whereas the more expensive ranibizumab is used for patients who can afford it or have some form of insurance cover.
Counterfeit bevacizumab containing 3% polyethylene glycol, citrate, ethanol and positive for a foreign body identified as Scytalidium sp. [9] has been reported. It has been suggested that the presence of considerable amount of endotoxin is responsible for causing endophthalmitis. This was termed as endotoxin-induced ocular toxic syndrome after an outbreak in patients undergoing intravitreal injection with counterfeit bevacizumab at a public hospital in Shanghai in China in September 2010. [10]
Ophthalmologists contacted us to conduct this analysis when they encountered ocular reactions after the use of suspected counterfeit bevacizumab. A suspected counterfeit sample was received in October 2014 from Hyderabad, and other samples were received in November 2014 from Kolkata and Delhi. All suspected samples were analysed with the identification protocol followed by Palmer in 2014 with modifications and additional protocols for quality assessment along with the samples. [4] Bradford analysis showed the absence of protein in 5 of 6 suspected counterfeit vials (83%). To widen the search and to fingerprint similarities among genuine formulations and to compare and contrast with counterfeit ones, we included other vials received from patients for dispensing into ampoules. Two personnel who were unaware of the genuineness of the samples did simple experiments such as frothing and alcohol precipitation tests before the use of vials of bevacizumab in clinics.
The commonest finding after the use of ′spurious′ bevacizumab was culture-negative endophthalmitis following intravitreal injections and the presence of anterior chamber reactions, hypopyon and vitritis. [10] Similar observations were made by Wang et al. in 2013, [11] who found endotoxin levels to be16 times higher at 280 mOsM/kg. We observed that among the 5 counterfeit vials, 2 had osmolarity between 253 and 261 mOsM/kg but the remaining 3 had osmolarity of 0, 41 and 129 mOsm/kg. The samples with higher osmolarity positively correlated for the presence of chloride when tested by the silver nitrite method.
Results of UV spectrophotometry suggested that it could be a quick method to check the presence of protein and could be correlated to the presence or absence of IgG in the samples. We could not quantify levels of endotoxins due to lack of adequate samples. However, we found that the frothing and alcohol-induced precipitation tests could be used to confirm the absence of protein in counterfeit vials.
As lack of protein is the most common observation in all counterfeit samples throughout the world, a quick one-drop experiment in surgical spirit (70% alcohol) would help ophthalmologists in ascertaining the purity of bevacizumab before its use. Although, running a gel electrophoresis is an appropriate assay to confirm IgG, it may not be available at all locations. Moreover, multiple sampling from an unpreserved vial will increase the chances of contamination of the bevacizumab solution. However, the alcohol precipitation test cannot differentiate between bevacizumab and any other protein which may have been added to the counterfeit preparations. The test will have no utility if the counterfeit sample contains any other protein instead of bevacizumab. In case the counterfeit protein is a mixture of a 25 and 50 kD protein even gel electrophoresis will not be useful. Only a detailed mass spectroscopy analysis will detect such differences.
To conclude, we analysed suspected counterfeit samples to compare and contrast these from genuine samples. Our study showed that lack of protein content is the most common feature of counterfeit bevacizumab in India as it is in other parts of the world. A simple frothing and alcohol precipitation test may be done in the clinic before administering bevacizumab to avoid ocular complications.
| 1. | Velpandian T, Sharma C, Garg SP, Mandal S, Ghose S. Safety and cost-effectiveness of single dose dispensing of bevacizumab for various retinal pathologies in developing countries. Indian J Ophthalmol 2007; 55: 488-90. [Google Scholar] |
| 2. | USFDA. Counterfeit version of avastin in U.S. distribution. Available at www.fda.gov/Drugs/DrugSafety/ucm291960.htm (accessed on 24 Nov 2014). [Google Scholar] |
| 3. | USFDA. Health care provider alert: Another counterfeit cancer medicine found in United States. Available at www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/ucm338283.htm (accessed on 24 Nov 2014). [Google Scholar] |
| 4. | Palmer E. Pfizer, Glaxo, others give Interpol money to fight counterfeiters. Available at www.fiercepharmamanufacturing.com/story/pfizer-glaxo-others-give-interpol-money-fight-counterfeiters/2013-03-12 (accessed on 24 Nov 2014). [Google Scholar] |
| 5. | Bridget MK. FDA warning: New batch of fake bevacizumab found. JAMA 2013; 309: 864. [Google Scholar] |
| 6. | Mackey TK. An exploration of counterfeit medicine surveillance strategies guided by geospatial analysis: Lessons learned from counterfeit avastin detection in the US drug supply chain. BMJ Open 2014; 4: e006657. [Google Scholar] |
| 7. | Blackstone EA, Fuhr JP Jr, Pociask SA. The health and economic effects of counterfeit drugs. Am Health Drug Benefits 2014; 7: 216-24. [Google Scholar] |
| 8. | Sato D.[Counterfeit medicines--Japan and the world]. Yakugaku Zasshi 2014; 134: 213-22. [Google Scholar] |
| 9. | Garcia-Aguirre G, Vanzinni-Zago V, Quiroz-Mercado H. Growth of Scytalidium sp. in a counterfeit bevacizumab bottle. Indian J Ophthalmol 2013; 61: 523-5. [Google Scholar] |
| 10. | Entezari M, Ramezani A, Ahmadieh H, Ghasemi H. Batch-related sterile endophthalmitis following intravitreal injection of bevacizumab. Indian J Ophthalmol 2014; 62: 468-71. [Google Scholar] |
| 11. | Wang F, Yu S, Liu K, Chen FE, Song Z, Zhang X, et al. Acute intraocular inflammation caused by endotoxin after intravitreal injection of counterfeit bevacizumab in Shanghai, China. Ophthalmology 2013;120:355-61. [Google Scholar] |
Fulltext Views
2,256
PDF downloads
1,005




