Translate this page into:
Haemophagocytic lymphohistiocytosis post-ChAdOx1 nCoV-19 vaccine: A rare case
Correspondence to ROBIN CHOUDHARY; robinch19@gmail.com
[To cite: Marwah V, Choudhary R, Kumar TA, Sharma M. Haemophagocytic lymphohistiocytosis post-ChAdOx1 nCoV-19 vaccine: A rare case. Natl Med J India 2024;37:90–2. DOI: 10.25259/NMJI_MS_466_21]
Abstract
The severe acute respiratory syndrome coronavirus 2 pandemic started in December 2019, spread like wildfire and took an immense toll on human life. ChAdOx1 nCoV-19 vaccine was used worldwide for the prevention of Covid-19. Covid-19 has been implicated in the causation of severe haemophagocytic lymphohistiocytosis (HLH) syndrome. However, the same has not been reported with ChAdOx1 nCoV-19 vaccine in the literature. We report a young man who developed secondary HLH post-ChAdOx1 nCoV-19 vaccination.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) primarily affects the lung but can also have severe systemic manifestation in the form of cytokine storm syndrome. Haemophagocytic lymphohistiocytosis (HLH) is a severe, life-threatening inflammatory syndrome associated with intense cytokine release, which has been implicated in Covid-19 disease. Covishield (ChAdOx1 nCoV-19) is a recombinant coronavirus vaccine that consists of recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 spike (S) glycoprotein. It was widely used in various countries including India, England, Brazil and South Africa. Hyperferritinaemic HLH has not been reported previously following administration of the vaccine. We describe a patient who developed severe cytokine release-related HLH following administration of the ChAdOx1 nCoV-19 vaccine.
THE CASE
A 40-year-old man, non-smoker, with no known comorbid conditions presented with a history of insidious onset, intermittent fever of 2 weeks to our hospital which had started 48 hours after vaccination with ChAdOx1 nCoV-19 vaccine. There was a history of occasional dry cough for 1 week. On examination, he had fever (103.7 °F), tachycardia (heart rate 110/minute) and a spleen palpable 2 cm below the costal margin. He had anaemia (haemoglobin 10.8 g/dl), lymphopenia (total lymphocyte count 2800/cmm), deranged liver enzymes (aspartate aminotransferase/alanine aminotransferase 171/202 i.u./L), hyponatraemia (121 mEq/L), raised triglyceride (320 mg/dl), serum fibrinogen (410 mg/dl) and lactate dehydrogenase (LDH) levels (926 i.u./L) (Table I). The C-reactive protein and ferritin levels (>1200 ng/ml on two occasions 7 days apart) were raised.
| Investigation | 9 March 2021 | 11 March 2021 | 12 March 2021 | 13 March 2021 | 14 March 2021 | 16 March 2021 | 17 March 2021 | 20 March 2021 |
|---|---|---|---|---|---|---|---|---|
| Haemoglobin (g/dl)/PCV (%) | 10.8/31 | 10.3/29 | 10.6/31.8 | 11.1/31.3 | 10.7/30.7 | 11.3/33 | 11.0/32 | 11.3/33.6 |
| TLC/cmm | 2800 | 1600 | 1600 | 2200 | 2200 | 3600 | 3500 | 7700 |
| DLC (%) (P/L/M/E) | 58/38/2/2 | 39/50/6/5 | 31/61/7/3 | 40/47/7/6 | 36/52/5/7 | 58/30/6/6 | 55/32/9/4 | 70/86/4/4 |
| Platelets/cmm | 90 000 | 98 000 | 145 000 | 207 000 | 215 000 | 280 000 | 268 000 | 275 000 |
| Urea (mg/dl) | 2 1 | 1 4 | 1 7 | 1 6 | 1 8 | 1 8 | 1 2 | 2 9 |
| Creatinine (mg/dl) | 1.0 | 1.0 | 0.8 | 0.9 | 0.8 | 1.0 | 0.8 | – |
| Na+/K+(mEq/L) | 121/3.6 | 131/3.5 | 135/4.1 | 136/3.6 | 137/3.9 | 138/4.0 | 136/3.8 | 136/4.4 |
| AST (i.u./L) | 171 | 160 | 124 | 113 | 9 6 | 7 4 | 6 4 | 4 2 |
| ALT (i.u./L) | 202 | 216 | 197 | 206 | 187 | 174 | 172 | 115 |
| LDH (i.u./L) | 926 | 858 | 771 | 729 | 662 | 499 | 570 | 513 |
PCV packed cell volume AST aspartate aminotransferase ALT alanine aminotransferase LDH lactate dehydrogenase TLC total leucocyte count DLC differential leucocyte count P/L/M/E neutrophils/lymphocytes/monocytes/eosinophils
His creatine phosphokinase and creatine kinase-MB levels were 361 i.u./L and 18 i.u./L, respectively. His prothrombin time and international normalized ratio (INR) were normal. His routine blood, urine, stool and sputum cultures were negative. The tropical disease work-up consisting of malaria, dengue, widal and scrub typhus and the virus panel consisting of HIV, Epstein–Barr virus, cytomegalovirus, hepatitis B and C virus were negative. His throat swab for reverse transcription-polymerase chain reaction for SARS-CoV-2 was done twice, which was negative. His throat swab was also sent for detection of other respiratory viruses such as influenza A and B, respiratory syncytial virus, rhinovirus, adenovirus, human metapneumo-virus and poxvirus, which were also negative. The peripheral blood smear showed normocytic, normochromic anaemia, leucopenia and features of activated lymphocytes with some of the lymphocytes showing ballerina skirt appearance. Ultrasound of the abdomen showed splenomegaly (15.4 cm). Computed tomogram of the chest showed few enlarged right paratracheal, pre-vascular and subcarinal lymph nodes (Fig. 1). He was managed conservatively, but he remained febrile. He underwent bone marrow aspiration and biopsy, which showed anaemia, lymphopenia with hypercellular marrow and no evidence of lymphoma or leukaemia. The endobronchial ultrasound-guided transbronchial needle aspiration of the mediastinal lymph node showed reactive lymphadenopathy with no evidence of lymphoma (Fig. 2). In view of raised markers of hyperinflammation, we thought of Covid-19 vaccine-related hyperinflammatory syndrome (HLH syndrome) and he was started on injectable steroids. His Covid-19 antibodies titres were positive. He showed good response and became afebrile in 48 hours. He was kept on tapering doses of oral steroids and he remained asymptomatic.
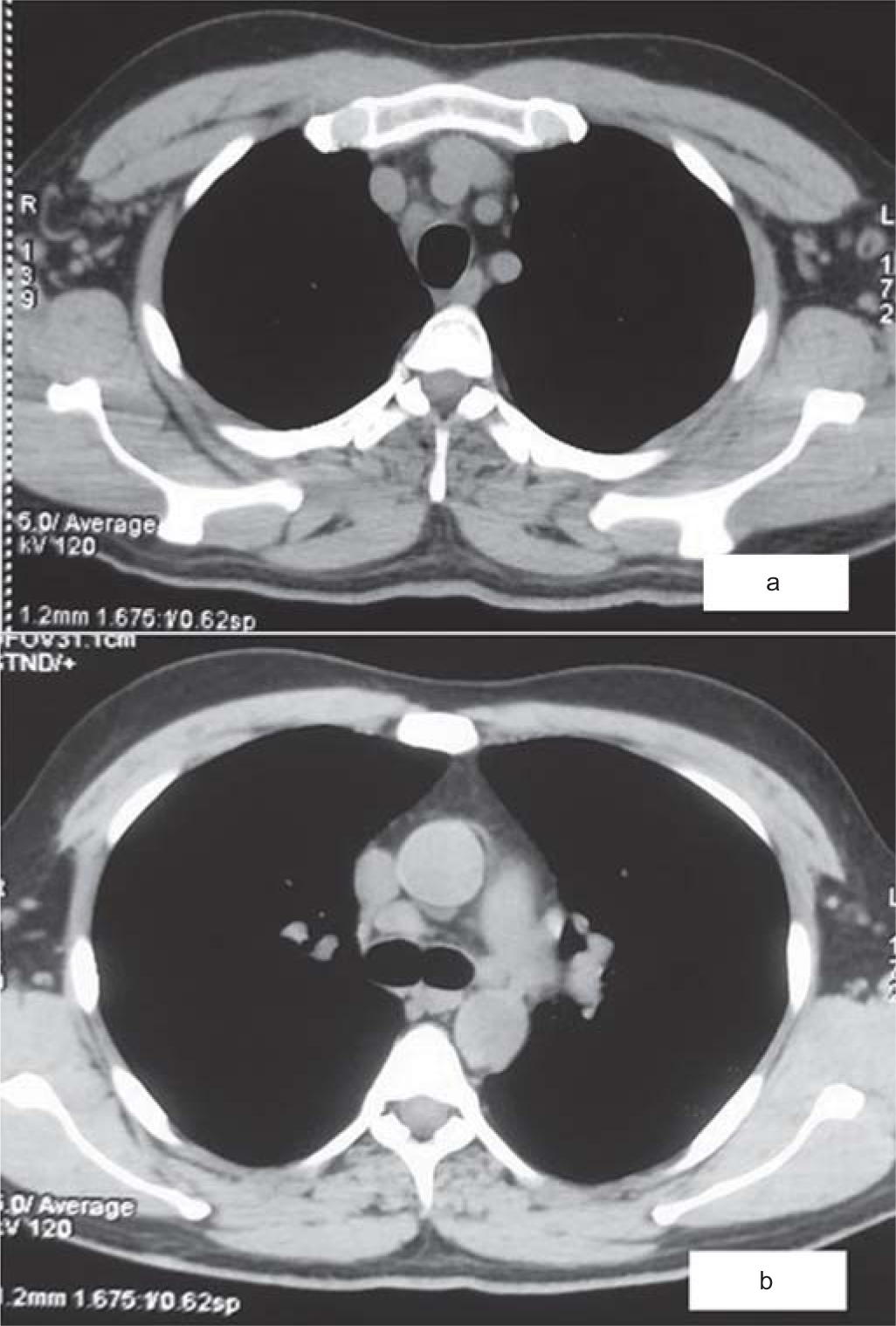
- (a and b) High-resolution computed tomogram of the chest (mediastinal window) showing paratracheal and subcarinal mediastinal lymphadenopathy

- Mediastinal lymph node aspiration cytology smear (MGG, ×400); cellular smear showing polymorphous population of small, mature lymphocytes (red arrow), small, cleaved centrocytes (black arrow), large centroblasts (green arrow) and immunoblasts (yellow arrow)
DISCUSSION
HLH is a rare and rapidly progressive systemic inflammatory disorder of uncontrolled immune activation characterized by cytopenia, excessive cytokine production and hyperferritinaemia. The common clinical features of HLH include acute unremitting fever, lymphadenopathy, hepatosplenomegaly and features of multi-organ failure. Due to a massive cytokine release, this clinical condition is considered as a cytokine storm syndrome. HLH can be primary or secondary. The primary form is seen due to various genetic mutations also called as familial HLH, which affects the cytotoxic function of T lymphocytes and natural killer (NK) cells and typically presents in children while secondary HLH may be caused due to a malignant, infectious or autoimmune/autoinflammatory causes.1–3 HLH can be diagnosed on the basis of various clinical and laboratory parameters though the presenting symptoms can be non-specific requiring a high index of suspicion. The 2004 Histiocyte Society criteria have been extensively used in diagnosing HLH.3 These include molecular testing consistent with HLH or five of eight of the following criteria: fever, splenomegaly, cytopenias affecting at least two lineages, hypertriglyceridaemia and/or hypofibrinogenaemia, haemophagocytosis (in bone marrow, spleen or lymph node), hyperferritinaemia, impaired NK cell function, and elevated soluble CD25 (sCD25) (i.e. sIL2R). Other common findings include transaminitis, coagulopathy, hyponatraemia, oedema, rash, hypoalbuminaemia, elevated LDH, C-reactive protein and Ddimer, increased low-density lipoprotein, decreased high-density lipoprotein, elevated cerebrospinal fluid protein and cells and neurological symptoms ranging from focal deficits to altered mental status. This scoring system is specific for HLH in children and in adults, other scoring systems such as H score or Delphi study is commonly used. Our patient fulfilled the criteria of HLH and was diagnosed as secondary HLH due to Covid-19 vaccine. The treatment of HLH includes steroids, treatment of the underlying cause and other agents such as etoposide.2–4
Covid-19 infection has been implicated in causing hyper-inflammatory syndrome such as secondary HLH.1,4–9 The mechanism implicated is dysregulated macrophage activation and uncontrolled release of various cytokines and chemokines including interleukin-6. ChAdOx1 nCoV-19 vaccine has proven to be efficacious with a potential role to prevent severe disease in the individual. However, it has been implicated in causing immune-mediated thrombotic complications.10,11 Covid-19 vaccine has been rarely reported to cause secondary HLH and to our knowledge, there is just one case reported by Tang et al., but the patient mentioned in their study had underlying chronic Epstein–Barr virus infection and the vaccine used was Chinese inactivated Covid-19 vaccine, which was not present in our case and this might be the index case of secondary HLH post-ChAdOx1 nCoV-19 vaccine.12 The underlying mechanism can be related to use of viral subunit in the vaccine which can act as a trigger for abnormal activation of the immune system. It can lead to macrophage activation and cytokine release leading to secondary HLH. The treatment includes corticosteroids, interleukin-1 blocking agents such as anakinra and rituximab. Our patient was managed with injectable steroids and showed a good response to the therapy.
Conclusion
The Covid-19 vaccine was regarded as a beacon of hope, but it is not completely safe and can cause complications. This is probably the first report of a rare complication of Covid-19 vaccine.
Conflicts of interest
None declared
References
- Hemophagocytic lymphohistiocytosis in a patient with COVID-19 treated with tocilizumab: A case report. J Med Case Rep. 2020;14:187.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465-77.
- [CrossRef] [PubMed] [Google Scholar]
- How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908-14.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic lymphohistiocytosis: A review inspired by the COVID-19 pandemic. Rheumatol Int. 2021;41:7-18.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and a hemophagocytic lymphohistiocytosis-like syndrome in the absence of ARDS. Chest. 2020;158:A1007.
- [CrossRef] [Google Scholar]
- Haemophagocytic syndrome and COVID-19. Clin Rheumatol. 2021;40:1233-44.
- [CrossRef] [PubMed] [Google Scholar]
- Could hemophagocytic lymphohistiocytosis be the core issue of severe COVID-19 cases? BMC Med. 2020;18:214.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic syndrome and COVID-19. Respir Med Case Rep. 2020;31:101162.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage activation in COVID-19 patients in intensive care unit. J Med Cases. 2020;11:211-14.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARSCoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111.
- [CrossRef] [Google Scholar]
- Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202-11.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J Hematol Oncol. 2021;14:87.
- [CrossRef] [PubMed] [Google Scholar]




