Translate this page into:
Innovations to automate manual ventilation during Covid-19 pandemic and beyond
Corresponding Author:
Joseph L Mathew
Division of Pediatric Pulmonology, Advanced Pediatrics Centre, Postgraduate Institute of Medical Education and Research, Chandigarh 160012
India
joseph.l.mathew@gmail.com
| How to cite this article: Mathew JL. Innovations to automate manual ventilation during Covid-19 pandemic and beyond. Natl Med J India 2020;33:366-371 |
Abstract
Manual ventilation by compressing self-inflating bags is a life-saving option for respiratory support in many resource-limited settings. Previous efforts to automate manual ventilation using mechatronic systems were unsuccessful. The Covid-19 pandemic stimulated re-exploration of automating manual ventilation as an economically viable alternative to address the anticipated shortage of mechanical ventilators. Many devices have been developed and displayed in the lay press and social media platforms. However, most are unsuitable for clinical use for a variety of reasons. These include failure to understand the clinical needs, complex ventilatory requirements in Covid-19 patients, lack of technical specifications to guide innovators, technical challenges in delivering ventilation parameters in a physiological manner, absence of guidelines for bench testing of innovative devices and lack of clinical validation in patients. The insights gained during the design, development, laboratory testing and clinical validation of a novel device designated the ‘Artificial Breathing Capability Device’ are shared here to assist innovators in developing clinically usable devices. A detailed set of clinical requirements from such devices, technical specifications to meet these requirements and framework for bench testing are presented. In addition, regulatory and certification issues, as well as concerns related to the protection of intellectual property, are highlighted. These insights are designed to foster an innovation ecosystem whereby clinically useful automated manual ventilation devices can be developed and deployed to meet the needs associated with the Covid-19 pandemic and beyond.Introduction
Several patients with respiratory failure, who require mechanical ventilation, do not receive it due to lack of availability, accessibility or affordability. In such situations, manual ventilation is offered as an alternative.[1] Rhythmic manual compression of self-inflating bags (SIB) or bag–valve–masks (BVM) drive air (or air–oxygen mixture) into the lungs. Releasing the bag permits air to flow out passively, thereby simulating a respiratory cycle. Although manual ventilation can be life-saving, it is essentially an uncontrolled procedure and can be dangerous if performed by inexperienced personnel (which is often the case in resource-limited settings). In recent years, efforts to mechanize the process of manual ventilation met with limited success,[2],[3],[4] and no devices are available for clinical use. Since early 2016, I have been leading an interdisciplinary team, which designed, developed and bench-tested the ‘Artificial Breathing Capability Device’ (ABCD) as a cost-effective alternative to manual ventilation. The technical and clinical details of ABCD are described elsewhere.[5],[6],[7],[8]
The Covid-19 pandemic, and the panic associated with anticipated shortage of mechanical ventilators in developed[9],[10] as well as developing countries,[11] re-ignited interest in automating manual ventilation. In recent months, many such devices have been produced and widely publicised in the lay press and social media platforms.[12],[13],[14],[15],[16],[17],[18],[19],[20] However, for various reasons, most of these are unsuitable for clinical use in Covid-19 as well as other conditions. On the other hand, there is a need for devices automating manual ventilation to offer a life-saving option in clinical settings, even beyond the Covid pandemic. Besides enhanced safety and efficacy, automation has the potential to resolve the adverse humanitarian and ethical considerations associated with manual ventilation.[21],[22] The following insights (based on my experience with the development of ABCD) are shared with inventors, innovators and imitators to help them develop appropriate devices that can meet clinical needs.
Bouquets
The effort by engineers/technologists and industry personnel, driven by altruism, at considerable personal cost, despite the shortage of workforce and material resources and disruption of conventional supply chains (during the prolonged lockdown), is indeed commendable. Faced with such challenges, it is remarkable that working models or prototypes could be developed within days to weeks. The media hype around these devices raised hope in frontline healthcare workers, policymakers, healthcare administrators and the general public. Government and non-government organizations supported these initiatives with liberal disbursement of funds, fast-tracked project approvals and soft loans to industries interested in mass production.
However, the following considerations need attention.
End-User Perspective
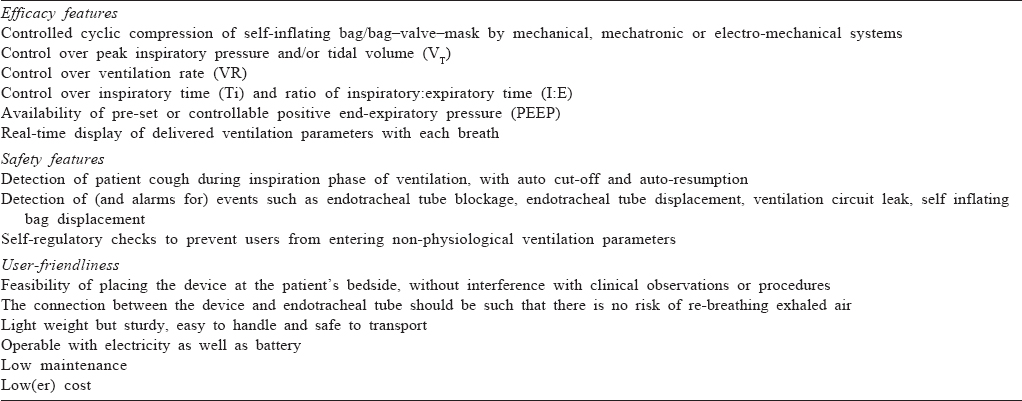
Most innovators focused on technology solutions to manual ventilation, concentrating efforts to mechanize the compression of SIB/BVM.[12],[13],[14],[15],[16],[17],[18],[19],[20] Limited attention was paid to clinical needs or perspectives of the end-users, namely physicians using the devices and the patients for whom the devices are intended. Many innovation teams did not even include any medical personnel. Thus, most of these devices merely automate SIB/ BVM compression at adjustable rates. Some offer additional features such as adjustable volume, variable inspiration time, capping of peak inspiratory pressure (PIP), insertion of positive end-expiratory pressure (PEEP) valve, display screens showing delivered parameters and audio/visual alarms when these parameters cross pre-set limits. In theory, these features appear appealing compared to completely uncontrolled manual ventilation. However, in practice, there are many gaps making them unsuitable for clinical use in patients. [Table - 1] summarizes the end-user requirements from devices automating manual ventilation. Attention to these perspectives will enhance the development of innovative devices.
Ventilation Needs in Covid-19 Patients
The ventilation needs of patients with Covid lung injury are complex. These include high oxygen demand, type I as well as type II respiratory failure, decreased lung compliance, acute respiratory distress syndrome, etc.[23],[24] The situation is more complex when cardiac injury is also involved. In these situations, manual ventilation is unlikely to be efficacious or safe. Therefore, devices that mechanize the process cannot meet the challenge. This is particularly true of devices whose technical capabilities for ventilation (in terms of pressure, volume, rate, inspiratory time and I:E ratio) are limited to supporting normal lungs.
Technical Specifications to Guide Innovators
At present, in India, there are no well-defined technical specifications to guide innovators developing devices for automating manual ventilation. This is one of the reasons for a slew of products that compress SIB/BVM but fail to meet the clinical need. A limited set of six criteria designated as ‘essential technical features for ventilators for Covid-19’ was prepared by the Defence Research and Development Organization at the end of March 2020 and readily accepted by the Ministry of Health and Family Welfare.[25]
However, these specifications are difficult to interpret. One of the six criteria[25] emphasizes that the device should be capable of providing invasive ventilation, non-invasive ventilation, and continuous positive airway pressure ventilation. However, no details were provided. Other criteria[25] demand the display of ‘lung mechanics’ and monitoring of ‘lung mechanics/inverse ratio (I:E)' without clarifying what these mean. There is also the somewhat strange criterion of ‘continuous working capability for 4–5 days’,[25] without mentioning how patients with Covid-19 would be managed beyond this limit. Thus, these specifications are inadequate to guide innovators to develop devices automating manual ventilation. In contrast, the United Kingdom Government Medicines and Healthcare Products Regulatory Agency lately published a detailed set of technical specifications expected from rapidly manufactured ventilator systems.[26] Similar but non-regulatory specifications were shared by a prestigious American university as well.[27] Around mid-May 2020, detailed technical specifications were laid down for the development of intensive care unit (ICU) ventilators in India,[28] but there was no guidance for devices automating manual ventilation. Based on a detailed understanding of the clinical needs (described above), I propose a reasonable set of technical specifications shown in [Table - 2].

Technical Challenges in Developing Devices to Automate Manual Ventilation
The respiratory cycle in mechanical ventilators involves a rapid rise in inspiratory pressure until the PIP is achieved, a pressure plateau for the duration of inspiration, followed by a fall to the pre-set PEEP level. PEEP is required to prevent the alveoli from collapsing. Mechanical ventilators allow all these components to be adjusted as per the clinical needs. On the other hand, manual ventilation is associated with a rapid rise in pressure and rapid fall to a zero level, without maintaining a plateau pressure during inspiration and without maintaining PEEP during expiration. This causes alveoli to collapse during expiration, necessitating higher opening pressure in the next breath. It is well documented that manual ventilation is associated with the delivery of far higher pressure (and the risk of barotrauma) compared to mechanical ventilators.[29] Unfortunately, most devices that mechanize SIB/BVM merely replicate this non-physiological pattern. Hence, there is a steep but transient rise in pressure (to the pre-set PIP) when the bag is compressed, followed by a rapid fall to the baseline, even if the bag remains compressed. In this situation, the true inspiratory time lasts for only 10%–15% of the set inspiratory time, and the remainder effectively contributes to the expiratory time. [Figure - 1] highlights this concept comparing the pressure profile delivered by one of the recently developed automated devices to the pressure profile delivered by ABCD.
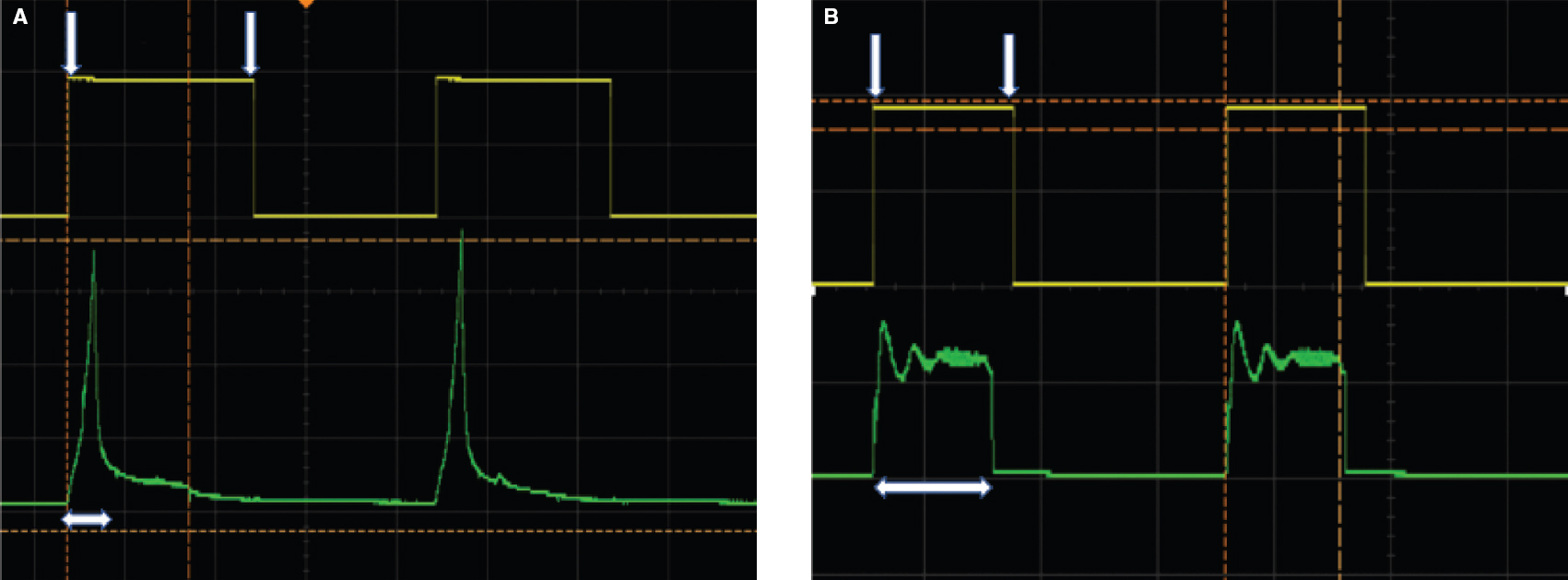 |
| Figure 1: Pressure profile delivered by an automated manual ventilation device (A) compared to Artificial Breathing Capability Device (B). The interval between the downward pointing white arrows represents inspiratory time of 1 second. The green tracing represents the pressure profile. The horizontal white arrows represent the effective inspiratory time |
Manual ventilation is performed by directly connecting the SIB/BVM to the endotracheal tube and placing it next to the patient’s head. This is not possible with a mechanical device, which has to be placed at some distance from the patent’s head. This necessitates the use of a long ventilation tube (usually at least 100 cm in length). If a single tube is used to connect the device to the endotracheal tube, exhaled air may get released into the tube and re-breathed in the next cycle. If separate inspiratory and expiratory tubings are used, an additional valve is required to prevent air flowing from the device (during inspiration) from blowing off through the expiratory limb (without reaching the patient). This problem can be overcome by detaching the flap membrane valve of the SIB and placing it in a separate casing just outside the endotracheal tube, thus preventing re-breathing of exhaled air.
Some automated devices require several breaths for the desired ventilation parameters to be delivered. This lag (up to 45 seconds in some devices) is unacceptable in clinical settings because the patients remain hypo-ventilated during the lag time.
Balancing Efficacy and Safety
Most innovative breathing devices focus on efficacy (i.e. compressing the SIB/BVM effectively), without a concomitant emphasis on patient safety. This is partly because innovators approach the problem from a technological, rather than clinical angle. Manual ventilation poses risks to patient safety by hypo-ventilation, hyper-ventilation, barotrauma or volutrauma, dys-synchrony with patient events, especially cough, endotracheal tube block, etc. In such situations, manual ventilation is not only ineffective but can be dangerous. Therefore, mere mechanization of the process carries the same risks. It can pose additional risks since the personnel performing manual ventilation intuitively adjust their hand movements when faced with such situations, whereas a machine cannot. Thus, a life-saving device can become life-threatening. These problems can be resolved by meticulous consideration of the clinical needs and designing devices to be fail-safe.
Laboratory Testing
Needless to mention, novel respiratory support devices require rigorous laboratory testing to ensure robustness, reliability and precision. This necessitates a laboratory environment to test the device in a variety of simulated clinical conditions, hi-tech data acquisition systems for continuous processing of data during the testing phase, and dedicated workforce to conduct the tests. Although there are specific standards for bench validation of such devices,[30] there are no guidelines for laboratory testing. Most innovators have conducted rudimentary tests on their prototypes, focusing on delivering set ventilation parameters for short periods. Based on the bench-testing of ABCD, a set of laboratory validation criteria are summarized in [Table - 3]. Testing may be done on a standard test lung (with the facility to vary compliance) or a clinical simulator. Testing should be done inputting various permutations and combinations of the parameters that can be set in the device.

Clinical Validation
The guiding principle of primum non nocere (first do no harm) in healthcare delivery has been forgotten or ignored by innovators of many respiratory devices. Therefore, most of these devices have been showcased (in the lay press and social media platforms) without clinical validation. Some of these devices have even been put to clinical use with disastrous results.[31],[32],[33] Clinical validation is complex, expensive and time-consuming, because it involves meticulous patient management, with continuous clinical as well as electronic monitoring to ensure patient safety during the testing phase. Although all innovators appear to appreciate this, most believe that it is outside the scope of their work (expecting someone else to do it). Many mistakenly believe that the Covid emergency situation justifies bypassing this step in the eagerness to do something rather than nothing. For a life-saving device that can be potentially life-threatening, clinical validation may require a step-wise approach starting with testing in terminally ill patients, followed by carefully selected salvageable patients, followed by pragmatic trials in unfiltered patients.
Regulatory and Certification Issues
In the absence of a functional Medical Devices Regulatory Authority, automated manual ventilation devices require to comply with the Bureau of Indian Standards and Central Drugs Standard Control Organization (CDSCO) guidelines. Citing the exigency of the Covid-19 pandemic, the CDSCO permitted manufacturers to produce ventilator devices without requiring any licensing.[34]
Intellectual Property Issues
Against the backdrop of the Covid-19 emergency, many innovators have ignored or completely violated intellectual property (IP) rights in the interest of producing something to meet the challenge. Thus, many prototypes that are improvizations or imitations of existing designs for automated devices are falsely claimed as novel innovations or even inventions. Further, many developers of these devices, being fully aware that there is no scope of claiming IP, freely disclose their prototypes to the lay press. This creates a piquant situation wherein genuine inventors and innovators are unable to disclose their work (until IP is protected), whereas improvisers and imitators do so. This poses the additional risk that genuine innovations will never receive IP protection, as the imitated designs have been widely published. Innovators working in developed countries are fortunate to have systems for fast-tracked IP protection, which is lacking in most developing countries.
Innovation Paradigm and Innovation Ecosystem
These insights are not intended to discourage innovation, but to develop a rational pathway that ultimately benefits all stakeholders (including healthcare consumers, providers, payers, policy-makers, etc.) and the healthcare system as a whole. The ideal paradigm of innovation (intended for clinical use) requires multiple steps starting from the bedside (to assess the clinical needs as mentioned previously), bench-work for development, followed by laboratory testing of prototypes, referring back to the bedside for clinical validation, followed by submission for regulatory approvals and certification. Only then should a product be commercialized and released in the market. Thereafter, ongoing post-marketing surveillance is essential to receive end-user feedback and identify issues affecting safety and efficacy. Thus, developing an innovative product resembles a journey more than a destination. Unfortunately, many innovators have short-circuited these logical steps.
It is impossible for a single individual or team to complete all the steps. This necessitates an innovation ecosystem that networks individuals and institutions with expertise in healthcare delivery, technology development, clinical validation supervised by ethics boards, IP protection, product realization, regulatory approval, commercialization, technology transfer, post-marketing surveillance and last (but not the least) securing funding for these activities. India is fortunate to have a national Biomedical Instruments and Devices Hub (https:// biomedhubpgichd.com), which has been established to address many of these needs. This hub, based at the Post-graduate Institute of Medical Education and Research (PGIMER) Chandigarh, works in collaboration with multiple technology institutions, healthcare institutions, industry partners and individual experts across multiple disciplines to facilitate innovators to navigate the innovation paradigm, providing (individual and/or institutional) support for each of the components involved.
Current Status of Innovative Respiratory Support Devices
At present, none of the innovative devices other than ABCD meets these standards.[35] However, the development of ABCD shows that it is achievable. In the interim, conventional ventilators that provide safety and efficacy should continue to be prioritized for development at low(er) cost.
The Last Word
As an individual, I applaud the innovative spirit, motivation to work in the public interest, and generous contributions of innovators attempting to mitigate the problems posed by Covid. As a clinician with some experience and expertise in ventilation, I urge innovators to carefully consider the insights shared in this aticle. As a fellow innovator, I welcome collaboration across disciplines, following all steps of the innovation pathway, to build usable devices with potential for use during the Covid pandemic and beyond. As a Coordinator of the Biomedical Instruments and Devices Hub, I offer its facilities and services towards one or more steps of design, development, laboratory testing and clinical validation of innovative respiratory support devices.
| 1. | Maurya PK, Kalita J, Paliwal VK, Misra UK. Manual AMBU ventilation is still relevant in developing countries. QJM 2008;101:990–1. [Google Scholar] |
| 2. | Ambu-bag Automation System and Method. International Application Published Under The Patent Cooperation Treaty (PCT). Available at www.patentimages. storage.googleapis. com/0d/5b/3c/2e57418ee200d3/WO2011022112A3.pdf (accessed on 30 Mar 2020). [Google Scholar] |
| 3. | Ambu Bag Automation System and Method. United States Patent Application Publication. Available at www.patentimages.storage.googleapis.com/d8/ce/df/f378c200595e3f/US20110041852A1.pdf (accessed on 26 Mar 2020). [Google Scholar] |
| 4. | Al Husseini AM, Lee HJ, Negrete J, Powelson S, Servi AT, Slocum AH, et al. Design and prototyping of a low-cost portable mechanical ventilator. J Med Device 2010;4:027514. [Google Scholar] |
| 5. | Mathew JL, Sharma M, Kumar N, Sukesha. Artificial Breathing Capability Device (ABCD): A Novel Life-saving Device for Resource-limited Settings. Presentation at 4th Global Forum on Medical Devices, Visakhapatnam, 12–14 December 2018. Available at www.who.int/medical_devices/global_forum/73_Artificial_ Breathing_Capability_Device_novel_life_saving_device_limited_resources.pdf (accessed on 17 May 2020). [Google Scholar] |
| 6. | WIPO IP Portal. WO2019229776-Automated Artificial Breathing Device. Publication Number WO/2019/229776. Available at www.patentscope.wipo.int/ search/en/detail.jsf?docId=WO2019229776&_cid=P11-KACLNC-27872-1 (accessed on 12 Jan 2020). [Google Scholar] |
| 7. | Mathew JL, Mathew TL. Invention, innovation, and imitation in India––necessity arising from the COVID-19 pandemic. Ann Natl Acad Med Sci (India) 2020;1: 1–9. [Google Scholar] |
| 8. | Artificial breathing capability device. Available at www.youtube.com/watch?v=UuzRc6pr4PM (accessed on 30 May 2020). [Google Scholar] |
| 9. | Truog RD, Mitchell C, Daley GQ. The toughest triage allocating ventilators in a pandemic. N Engl J Med 2020;382:1973–5. [Google Scholar] |
| 10. | Koonin LM, Pillai S, Kahn EB, Moulia D, Patel A. Strategies to inform allocation of stockpiled ventilators to healthcare facilities during a pandemic. Health Secur 2020;8:69–74. [Google Scholar] |
| 11. | Bora G. Rising Covid-19 cases scare hospitals with insufficient ventilators. Available at www.economictimes.indiatimes.com/small-biz/sme-sector/covid-19-ventilators-hospitals-agva-healthcare-coronavirus/articleshow/ 74840459.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst (accessed on 15 May 2020). [Google Scholar] |
| 12. | Indian Institute of Technology, Kanpur: Standard Chartered Bank First Entity to Fund Ventilator Prototype by IIT Kanpur. Available at www.business-standard.com/article/news-ani/indian-institute-of-technology-kanpur-standard-chartered-bank-first-entity-to-fund-ventilator-prototype-by-iit-kanpur-120040700345_1.html (accessed on 16 May 2020). [Google Scholar] |
| 13. | BW Online Bureau. IIT Hyderabad CfHE-incubated startup develops low-cost and portable emergency use ventilator. Available at www.bweducation. businessworld.in/article/IIT-Hyderabad-CfHE-Incubated-startup-develops-low-cost-portable-emergency-use-ventilator-/03-04-2020-188175 (accessed on 16 May 2020). [Google Scholar] |
| 14. | Watch: How the World’s Lowest-Cost “Made in India” Ventilator. Available at www.timesofindia.indiatimes.com/gadgets-news/watch-how-the-worlds-lowest-cost-made-in-india-ventilator-works-without-electricity/articleshow/75243170.cms (accessed on 17 May 2020). [Google Scholar] |
| 15. | Indian Railways Develops Low-cost Ventilator “Jeevan”, Seeks ICMR Approval. Available at www.economictimes.indiatimes.com/industry/transportation/ railways/railways-develops-low-cost-ventilator-jeevan-seeks-icmr-approval/ articleshow/74996857.cms?from=mdr (accessed on 17 May 2020). [Google Scholar] |
| 16. | Nanda PK. IIT-Roorkee, AIIMS-Rishikesh develop low-cost portable ventilator. Available at www.livemint.com/news/india/covid-19-iit-roorkee-develops-low-cost-portable-ventilator-offers-to-industry-11585842779864.html (accessed on 17 May 2020). [Google Scholar] |
| 17. | Hyundai motor India develops automatic Ambu bag actuator prototype. Available at www.auto.hindustantimes.com/auto/news/hyundai-motor-india-develops-automatic-ambu-bag-actuator-prototype-41587208914044.html (accessed on 17 May 2020). [Google Scholar] |
| 18. | Rana C. Chandigarh: PGIMER doctor creates automatic AMBU ventilator. Available at www.indianexpress.com/article/cities/chandigarh/chandigarh-pgimer-doctor-creates-automatic-ambu-ventilator-6332803 (accessed on 31 Mar 2020). [Google Scholar] |
| 19. | Karnataka based startup comes up with automatic ambu bag. Available at www.newindianexpress.com/states/karnataka/2020/apr/05/karnataka-based-startup-comes-up-with-automatic-ambu-bag-2126133.html (accessed on 17 May 2020). [Google Scholar] |
| 20. | Khanna B. Ventilator that can treat two patients, courtesy IIT Ropar scientists. Available at www.timesofindia.indiatimes.com/home/education/news/ ventilator-that-can-treat-two-patients-courtesy-iit-ropar-scientists/ articleshow/74967790.cms (accessed on 17 May 2020). [Google Scholar] |
| 21. | Barsky E, Sayeed S. Parental manual ventilation in resource-limited settings: An ethical controversy. J Med Ethics 2020;46:459–64. [Google Scholar] |
| 22. | Halpern P, Dang T, Epstein Y, Van Stijn-Bringas Dimitriades D, Koenig KL. Six hours of manual ventilation with a bag-valve-mask device is feasible and clinically consistent. Crit Care Med 2019;47:e222–6. [Google Scholar] |
| 23. | Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Wen SD, Phua GC, et al. Rapid progression to acute respiratory distress syndrome: Review of current understanding of critical illness from Covid-19 infection. Ann Acad Med Singapore 2020;49:108–18. [Google Scholar] |
| 24. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. [Google Scholar] |
| 25. | Essential technical features for ventilator for Covid-19. Available at www.mohfw.gov.in/pdf/EssentialTechfeaturesforVentilators.pdf (accessed on 12 May 2020). [Google Scholar] |
| 26. | Medicines and healthcare products regulatory agency (MHRA) recently published a detailed set of technical specifications expected from rapidly manufactured ventilator systems. document RMVS0001 specification. Available at www.assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/879382/RMVS001_v4.pdf (accessed on 22 May 2020). [Google Scholar] |
| 27. | MIT E-Vent MIT emergency ventilator. Key ventilation specifications. Available at www.e-vent.mit.edu/clinical/key-ventilation-specifications (accessed on 22 May 2020). [Google Scholar] |
| 28. | HLL Lifecare Limited. Request for proposal for supply of ventilator to GOI institutes. Available at www.lifecarehll.com/RFP-%20Ventilators%20(HLL)% 2014052020.pdf (accessed on 12 May 2020). [Google Scholar] |
| 29. | Peak pressures during manual ventilation. Respir Care 2005;50:340–4. [Google Scholar] |
| 30. | Medical Electrical Equipment part 2-80: Particular Requirements for Basic Safety and Essential Performance of Ventilatory Support Equipment for Ventilatory Insufficiency; 2018. Available at www.iso.org/standard/68844.html (accessed on 12 May 2020). [Google Scholar] |
| 31. | Singh R. Behind Ahmedabad’s ventilator controversy, a backstory of connections to top BJP leaders. Available at www.thewire.in/political-economy/modis-monogrammed-suit-rajkot-ventilator-vijay-rupani (accessed on 22 May 2020). [Google Scholar] |
| 32. | Outlook Web Bureau. Gujarat Govt Uses Controversial “Breathing Apparatus” as Ventilator; Hospital Says Not ‘High End’. Available at www.outlookindia.com/website/story/india-news-gujarat-govt-uses-controversial-breathing-apparatus-as-ventilator-hospital-says-not-high-end/353077 (accessed on 22 May 2020). [Google Scholar] |
| 33. | ‘Approved by Centrally Accredited Lab’: Gujarat Govt Defends Locally Developed ‘Dhaman-1’ Ventilators. Available at www.news18.com/news/india/approved-by-centrally-accredited-lab-gujarat-govt-defends-locally-developed-dhaman-1-ventilators-2628567.html (accessed on 21 May 2020). [Google Scholar] |
| 34. | Aggarwal A, Verma K, Sharma A. Helping India Breathe: Ventilator Manufacturing during Covid-19. Available at www.static.investindia.gov.in/2020-07/Helping%20India%20Breathe_0.pdf (accessed on 25 Jul 2020). [Google Scholar] |
| 35. | Mathew JL, Sharma M, Gawri A, Sukesha, Kumar N, Chander A, et al. Design, development and evaluation of Artificial Breathing Capability Device (ABCD): A novel innovation for respiratory support. BMJ Innov 2021;7:40–6. [Google Scholar] |
Fulltext Views
2,797
PDF downloads
1,331




