Translate this page into:
Intensive insulin therapy and plasma exchange in hypertriglyceridaemic acute pancreatitis with multiple organ dysfunction
Corresponding Author:
Kiran Kumar Gudivada
Department of Critical Care Medicine, St John's Medical College and Hospital, Bengaluru 560034, Karnataka
India
gkiran17medico@gmail.com
| How to cite this article: Gudivada KK, Krishna B. Intensive insulin therapy and plasma exchange in hypertriglyceridaemic acute pancreatitis with multiple organ dysfunction. Natl Med J India 2019;32:352-354 |
Abstract
Acute pancreatitis (AP) is a common emergency in gastroenterology. After gallstone disease and alcoholism, hypertriglyceridaemia (HTG) is the next common cause for AP. The role of intensive insulin therapy (IIT) and plasma exchange (PE) in hypertriglyceridaemic acute pancreatitis (HTG-AP) is still debatable. We report a 56-year-old farmer with HTG-AP who presented with a recurrence of AP. On admission, his plasma triglycerides were 4773 mg/dl with a wide range of laboratory abnormalities. Over the course of his illness, he developed multiple organ failure. He received early IIT initially, and PE once haemodynamic stability was achieved. This approach improved the functioning of the organs. In haemodynamically unstable patients with HTG-AP, we suggest early initiation of IIT, followed by adjuvant PE after the resolution of shock, to ameliorate organ dysfunction and improve overall outcome.Introduction
Nearly half the patients with acute pancreatitis (AP) require admission to an intensive care unit (ICU). The most common cause for AP is gallstone disease followed by alcohol consumption.[1] Although the literature from India is limited, in the western literature hypertriglyceridaemia (HTG) accounts for 1%–9% of all patients with AP and up to 56% of pancreatitis during pregnancy.[2],[3] HTG-AP is potentially lethal if not identified and treated early in the course of the disease. At present, there are no definitive guidelines for the management of HTG-AP. Plasma exchange (PE) is considered as a treatment modality for HTG-AP, but there is no conclusive evidence to show improvement in the overall morbidity or mortality.[4],[5],[6]
Intravenous insulin has been used to treat HTG-AP but in a limited number of cases.[7],[8],[9] The advantage of intensive insulin therapy (IIT) is that it can be safely administered in patients with haemodynamic instability. To the best of our knowledge, there is limited published literature on the management of patients with HTG-AP who present with shock. We present a patient with HTG-AP who was admitted to our ICU with multiple organ dysfunction and was treated with early IIT and followed by PE as an adjuvant therapy once haemodynamic stability was achieved.
Written informed consent was obtained from the patient before writing the manuscript.
The Case
A 56-year-old male was admitted to our ICU with a recurrence of pancreatitis after having a fatty meal. He had a past history of pancreatitis, after which he was started on tablet finofibrate 150 mg. He was also suffering from type 2 diabetes mellitus for 3 years and was on tablet metformin 500 mg twice a day. According to his family, he was non-compliant with these medications and his last alcohol consumption was 8 years back. His admission APACHE II score was 18 and the plasma triglycerides (TGs) were 4773 mg/dl. He was diagnosed with HTG-AP.
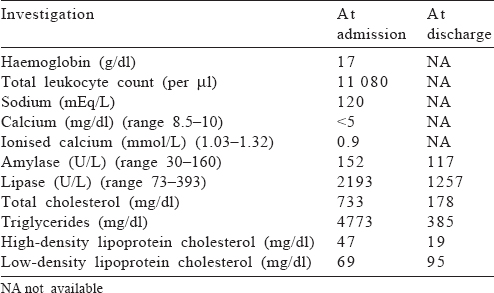
Over the next 6 hours, despite fluid resuscitation, he developed hypotension requiring vasopressors, oliguria and hypoxaemia, and hence, he was intubated. He had mixed acidosis with increasing serum lactate from 2.5 to 4.5 mmol/L and blood glucose from 380 to 450 mg/dl with +2 urine ketone bodies. Intravenous insulin infusion was started at 7 units/hour after a bolus of 7 units. Once the ketoacidosis was resolved, blood sugars were maintained between 110 and 180 mg/dl using insulin infusion and 5% dextrose. Other relevant investigations are shown in [Table - 1]. As this patient had refractory shock, PE was not considered for the first 2 days. After one session of haemodialysis with continued insulin infusion, his acidosis and haemodynamic status improved, vasopressors were stopped and TGs reduced to 2545 mg/dl on day 3. Since his plasma TGs were still high, two sessions of PE were done on ICU day 3 and 4, with each session consisting of 2 L of plasma removal and replacement with 8 units of fresh frozen plasma and 200 ml of 20% albumin. PE reduced the TG levels to 1260 mg/dl and 709 mg/dl after the 1st and 2nd sessions, respectively [Figure - 1]. Subsequently, the urine output improved and the patient was extubated on day 5. Insulin infusion was stopped on the same day. The patient received tablet finofibrate 150 mg b.d. and thromboprophylaxis with heparin (5000 i.u. subcutaneous b.d.) from day 2. He was discharged to the ward on day 6.
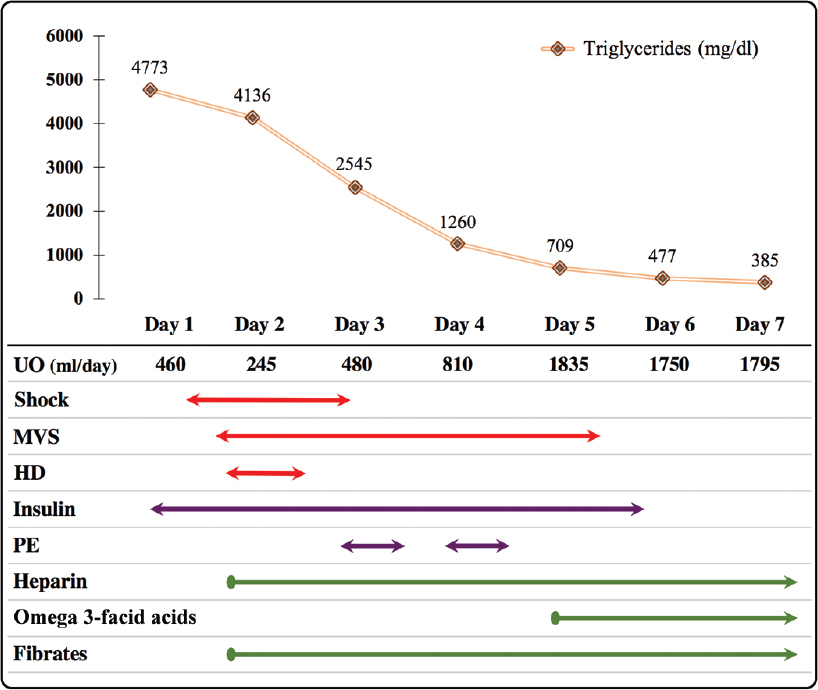 |
| Figure 1: Trend of triglyceride levels and temporality of therapeutic interventions received by the patient in the intensive care unit. UO urine output MVS mechanical ventilator support HD haemodialysis PE plasma exchange |
Discussion
Plasma TG level >1000 mg/dl is considered a risk factor for pancreatitis.[10] Our patient had consumed a fatty meal that could have caused a surge in TG levels and precipitated AP. His admission TG level (4773 mg/dl) was consistent with a diagnosis of HTG-AP.
Serum sodium, calcium and amylase levels are often misinterpreted in the presence of high TG levels. Low serum sodium (120 mEq/L) in this patient can be explained by the displacement of water molecules in the presence of high lipid levels. Sodium being an aqueous-phase ion can be spuriously low (pseudo-hyponatraemia) when measured by flame photometry or indirect potentiometry.[11] High TGs could also have interfered with the calorimetric reading of amylase (152 i.u.), thus misinterpreting it as a pseudo-normal value.[12] Hypocalcaemia ensues when calcium binds with excessive free fatty acid (FFA)–albumin complexes.[13]
PE is a therapy of choice for HTG-AP. PE decreases lipotoxicity by reducing FFAs, a breakdown product of TG.[14] However, timing of PE in HTG-AP is still unknown. Though some case reports mention beneficial effects of early PE in alleviating abdominal pain and organ dysfunction,[4],[15] a large observational study failed to show any survival benefit.[6] Moreover, early PE may not be feasible in patients presenting with refractory shock.
In our patient, PE was initiated about 48 hours after the onset of symptoms. This was intentional, as he had shock and PE that could further worsen his haemodynamics. IIT may be a safer alternative in these patients. Insulin at an infusion rate of 0.1–0.3 units/kg/hour is known to decrease TG levels by two mechanisms: (i) by enhancing the lipoprotein lipase activity, thereby promoting hydrolysis of TGs; and (ii) by improving lipase activity in adipose tissues.[16] In addition, insulin activates translocation of fatty acid transporter protein on adipocyte and promotes the uptake of FFA. This dual mechanism may explain the comparable efficacy of insulin with PE.[17],[18]
Compared to PE, IIT has additional advantages: it is less invasive, less expensive, can be initiated instantly, easy to monitor and avoids transfusion-related complications. On the other end, PE has several disadvantages: it is an invasive strategy, requires trained nursing staff and prompts the availability of advanced blood bank facilities.
Our patient presented with HTG and ketoacidosis. Insulin infusion was started to manage both the conditions. There was a rapid decline in TGs (4773 to 2545 mg/dl) and reduction in ketoacidosis with improvement in haemodynamic status. However, it is difficult to extrapolate the entire favourable effects to reduction in TG alone because there was simultaneous improvement in acidosis due to IIT and haemodialysis. Nevertheless, the magnitude of decline in TGs was considerable (46.7% reduction) after IIT, which could have reduced the inflammatory burden. Subsequent PE resulted in further reduction in TGs. There was an improvement in renal functions and respiratory mechanics as reflected by an increase in urine output and an ability to wean and extubate the patient by day 5. It is important to note that the IIT was continued during PE therapy. Even though PE is considered as a primary treatment, it has been recommended to not ignore the effect of IIT which had reduced the TG levels by 46.7% in this patient.
Our patient received heparin for thromboprophylaxis. Conflicting data exist for the therapeutic use of heparin in HTG, as it can deplete plasma lipoprotein lipase stores and paradoxically increase the chylomicrons.[19] Finofibrate was prescribed as an adjuvant therapy for long-term lipid control and secondary prevention of AP.
Conclusion
In a case of haemodynamically unstable HTG-AP where PE cannot be initiated, we recommend early treatment with IIT. We suggest initiation of PE as an additional therapeutic option once haemodynamic stability is achieved. This approach might be safer and more practical to ameliorate organ dysfunction and improve overall survival.
Conflicts of interest. None declared
| 1. | Trapnell JE, Duncan EH. Patterns of incidence in acute pancreatitis. Br Med J 1975;2:179–83. [Google Scholar] |
| 2. | Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology 2016;16:469–76. [Google Scholar] |
| 3. | Chang CC, Hsieh YY, Tsai HD, Yang TC, Yeh LS, Hsu TY, et al. Acute pancreatitis in pregnancy. Zhonghua Yi Xue Za Zhi (Taipei) 1998;61:85–92. [Google Scholar] |
| 4. | Chen JH, Yeh JH, Lai HW, Liao CS. Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis. World J Gastroenterol 2004;10:2272–4. [Google Scholar] |
| 5. | Furuya T, Komatsu M, Takahashi K, Hashimoto N, Hashizume T, Wajima N, et al. Plasma exchange for hypertriglyceridemic acute necrotizing pancreatitis: Report of two cases. Ther Apher 2002;6:454–8. [Google Scholar] |
| 6. | Gubensek J, Buturovic-Ponikvar J, Romozi K, Ponikvar R. Factors affecting outcome in acute hypertriglyceridemic pancreatitis treated with plasma exchange: An observational cohort study. PLoS One 2014;9:e102748. [Google Scholar] |
| 7. | Alagözlü H, Cindoruk M, Karakan T, Unal S. Heparin and insulin in the treatment of hypertriglyceridemia-induced severe acute pancreatitis. Dig Dis Sci 2006; 51:931–3. [Google Scholar] |
| 8. | Coskun A, Erkan N, Yakan S, Yildirim M, Carti E, Ucar D, et al. Treatment of hypertriglyceridemia-induced acute pancreatitis with insulin. Prz Gastroenterol 2015;10:18–22. [Google Scholar] |
| 9. | Rodríguez Santana Y, Nimo Román A, García Sáez I, López Alvarez JM, Consuegra Llapur E, González Jorge R, et al. Treatment of severe hypertriglyceridemia with continuous insulin infusion. Case Rep Crit Care 2011;2011:293917. [Google Scholar] |
| 10. | Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969–89. [Google Scholar] |
| 11. | Howard JM, Reed J. Pseudohyponatremia in acute hyperlipidemic pancreatitis. A potential pitfall in therapy. Arch Surg 1985;120:1053–5. [Google Scholar] |
| 12. | Wong EC, Butch AW, Rosenblum JL. The clinical chemistry laboratory and acute pancreatitis. Clin Chem 1993;39:234–43. [Google Scholar] |
| 13. | Warshaw AL, Lee KH, Napier TW, Fournier PO, Duchainey D, Axelrod L, et al. Depression of serum calcium by increased plasma free fatty acids in the rat: A mechanism for hypocalcemia in acute pancreatitis. Gastroenterology 1985;89: 814–20. [Google Scholar] |
| 14. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011;3:107ra110. [Google Scholar] |
| 15. | Kyriakidis AV, Raitsiou B, Sakagianni A, Harisopoulou V, Pyrgioti M, Panagopoulou A, et al. Management of acute severe hyperlipidemic pancreatitis. Digestion 2006;73:259–64. [Google Scholar] |
| 16. | Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 1989;320:1060–8. [Google Scholar] |
| 17. | He WH, Yu M, Zhu Y, Xia L, Liu P, Zeng H, et al. Emergent triglyceride-lowering therapy with early high-volume hemofiltration against low-molecular-weight heparin combined with insulin in hypertriglyceridemic pancreatitis: A prospective randomized controlled trial. J Clin Gastroenterol 2016;50:772–8. [Google Scholar] |
| 18. | Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2002;2:477–88. [Google Scholar] |
| 19. | Näsström B, Olivecrona G, Olivecrona T, Stegmayr BG. Lipoprotein lipase during continuous heparin infusion: Tissue stores become partially depleted. J Lab Clin Med 2001;138:206–13. [Google Scholar] |
Fulltext Views
2,252
PDF downloads
514




