Translate this page into:
Invasive fungal infection with a rare organism in a patient with acute myeloid leukaemia
Corresponding Author:
Mehmet Baysal
Department of Haematology, Trakya University Faculty of Medicine, Balkan Yerleskesi 22030, Edirne
Turkey
drmehmetbaysal@gmail.com
| How to cite this article: Baysal M, Ümit E, Ozdover AC, Kirkizlar O, Demir AM. Invasive fungal infection with a rare organism in a patient with acute myeloid leukaemia. Natl Med J India 2020;33:22-23 |
Abstract
Invasive fungal infections are a major cause for morbidity and mortality in patients with acute myeloid leukaemia (AML). Long duration of hospitalization and increased costs are secondary burdens for patients and caregivers. The clinical manifestations are variable with a spectrum of different organs or systems. Factors related with invasive fungal infections may be categorized as host-related including the underlying disease, treatment and colonization status and pathogen-related including the capacity of the microorganism for defence, growth, tolerance and tissue affinity. The diagnosis of invasive fungal infection is confirmed with histopathological or microbiological demonstration of the microorganism, and commonly treatments are based on probability rather than definitive diagnosis due to patients fragile conditions preventing interventions. We aimed to present the less frequent yet difficult-to-treat organism, Verticillium causing invasive fungal infection in a patient with AML undergoing remission induction therapy.Introduction
With the increase in treatment alternatives for haematological malignancies, the incidence of both superficial and invasive fungal infections (IFIs) has increased.[1] The spectrum of fungal infections ranges from local skin or soft tissue infections to disseminated or invasive infections of vital organs or systems. To understand the enemy, we have to know to classify fungi as yeasts and moulds. Fungi-forming yeasts include Candida spp., Cryptococcus spp. and Pneumocystis jiroveci. Moulds are fungi-forming filamentous structures developed within the environment and forming hyphal structures within tissues. Moulds include Aspergillus spp. and pathogens causing mucormycosis.[2],[3]
Besides their individual structures, mycoses are categorized based on the site of infection as superficial, subcutaneous or invasive-deep and based on the route of procurement as exogeneous or endogeneous. Routes of entry may be classified for exogenous as airborne and cutaneous, while endogeneous entry may include colonization or reactivation of a previous infection.
Verticillium spp. is usually found in vegetables and soil. These species have harmful effects on plants but in humans rarely cause infections.[4] Acute myeloid leukaemia (AML) patients undergoing remission induction therapy are more easily infected with fungal organisms. However, IFI prophylaxis with posaconazole has reduced the number of fungal infections.[5] Rare and challenging organisms cause compelling and hard-to-treat infection in AML patients. In this case report, we present an AML patient with Verticillium skin infection and keratitis.
The case
A 58-year-old male with complaints of fatigue was admitted to our hospital. Physical examination showed general pallor and occasional petechiael bleeding in the lower extremities. He had no history of chronic illness, was a non-smoker and did not report any illicit drug use. His laboratory evaluation showed anaemia, thrombocytopenia and neutropenia. Atypical mononuclear cells with fine chromatin, basophilic cytoplasm and two to three nucleolii were observed in his peripheral smear. Flowcytometry evaluation showed a monoclonal blastic population positive for CD13, CD33, CD34, CD117, MPO, HLA-DR, CD14 and CD64 and negative for CD2, CD3, CD4, CD8, CD19 and CD20. Bone marrow aspiration and biopsy showed a diffuse infiltration with atypical monocytoid blastic cells. Examination of 20 metaphases showed 46 XY karyotype. He was negative for FLT3, NPM1 and fluorescence in situ hybridization (FISH) analysis and also negative for t(8;21), t(15;17) and t(4;11). He was diagnosed with standard risk AML and 7+3 (cytrabine 100 mg/m2 continuous infusion for 7 days and idarubicin 12 mg/m2 for 3 days) treatment protocol was started with oral posaconazole 600 mg/day in suspension form for prophylaxis of IFI. The patient had refractory disease to 7+3 protocol and treatment was followed with FLAG-IDA (fludarabine, 30 mg/m2 intravenous [i.v.] for 4 days, cytarabine 2 g/m2 i.v. for 4 days, idarubicin 10 mg/m2 i.v. for 3 days and glycosylated G-CSF at a daily dose of 300 μg/m2, from day 1 until day 5 of treatment[6] and clofarabine 20 mg/m2 for 5 days and cytarabine 2000 mg/m2 for 5 days).[7]
On day 14 after the third-line remission induction treatment, the patient had neutropaenic fever. Broad-spectrum antibiotics with anti-pseudomonal activity were started. Fever persisted, and generalized macular, necrotic, round, 1 cm diameter skin lesions developed [Figure - 1]. Serum galactomannan antigen assay was positive, and CT scan of the thorax showed an increase in the density of ground-glass opacification bilaterally in the lower segments of the lungs. The patient was diagnosed with probable IFI and liposomal amphotericin B was started for treatment. Three days after the treatment, new skin lesions developed with bilateral conjunctivitis and keratitis [Figure - 2]. Verticillium spp. was detected in the cultured skin lesions as well as eye swap material. Voriconazole eye drops and voriconazole i.v. was were added to the treatment. However, the patient did not respond well to treatment and died because of sepsis on day 24 after chemotherapy.
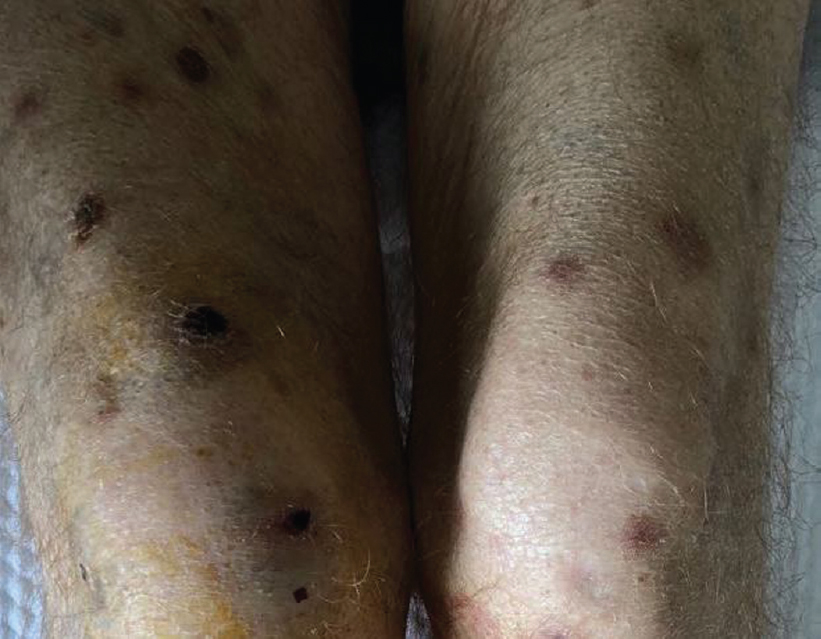 |
| Figure 1: Generalized macular, necrotic, round, 1-cm diameter skin lesions |
 |
| Figure 2: Bilateral conjunctivitis and keratitis |
Discussion
Verticillium species are characterized by filamentous hyaline septate with mould formation and a can be found widely in the environment.[4] The number of cases caused by Verticillium spp. in humans are limited. In the literature, a leukaemia patient with Verticillium spp. keratitis during immunocompromisation, two immunocompetent patients with Verticillium spp. keratitis and endophthalmitis, skin infections in a renal transplant patient and Verticillium peritonitis in a peritoneal dialysis patient have been reported.[8],[9],[10] Verticillium infections are scarce in humans and consist of case reports. In our patient, infection with this particular fungus was extensive on the skin as well as bilateral conjunctiva and cornea. In an immunocompromised patient, this infection may first have involved the skin and later been transmitted to the eyes by hand-to-eye contact. Therefore, careful examination of the patient and early recognition are crucial to control the disease. The patient was treated with three lines of remission induction therapy due to the acute refractoriness of disease. This treatment possibly increased the risk of fungal infection due to myelosuppression and immunosuppression.
Verticillium rarely causes infections in humans and treatment is usually difficult and has not been standardized. In published case reports, the treatment was usually an azole drug that was well tolerated and responsive. In our patient, the prophylactic posaconazole treatment was changed to liposomal amphotericin B, and both local and systemic voriconazole was also combined with amphotericin B to control the infection.
Breakthrough IFIs with rare organisms are emerging causes of difficult infections in AML patients. Every patient should be examined thoroughly for infectious foci and all possible lesions should be evaluated for agent isolation.
Conflict of interest. None declared
| 1. | Garnica M, da Cunha MO, Portugal R, Maiolino A, Colombo AL, Nucci M. Risk factors for invasive fusariosis in patients with acute myeloid leukemia and in hematopoietic cell transplant recipients. Clin Infect Dis 2015;60:875–80. [Google Scholar] |
| 2. | Arif S, Perfect JR. Emergence of the molds other than Aspergillus in immunocompromised patients. Clin Chest Med 2017;38:555–73. [Google Scholar] |
| 3. | Skiada A, Pavleas I, Drogari-Apiranthitou M. Rare fungal infectious agents: A lurking enemy. F1000Res 2017;6:1917. [Google Scholar] |
| 4. | Wu CJ, Chang TC, Lee HC, Chen TY, Lee NY, Chang CM, et al. Verticillium infection – A rare cause of hepatosplenic abscesses. Diagn Microbiol Infect Dis 2008;62:427–9. [Google Scholar] |
| 5. | Dragonetti G, Criscuolo M, Fianchi L, Pagano L. Invasive aspergillosis in acute myeloid leukemia: Are we making progress in reducing mortality? Med Mycol 2017;55:82–6. [Google Scholar] |
| 6. | Montesinos P, de la Rubia J, Orti G, Sanz J, Martinez D, Mendoza N, et al. FLAG-IDA regimen (Fludarabine, cytarabine, idarubicin and G-CSF) in the treatment of patients with high-risk acute myeloid leukemia and myelodysplastic syndromes. Blood 2007;110:2866. [Google Scholar] |
| 7. | Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: Results from the CLASSIC I trial. J Clin Oncol 2012;30:2492–9. [Google Scholar] |
| 8. | Shin JY, Kim HM, Hong JW. Keratitis caused by Verticillium species. Cornea 2002;21:240–2. [Google Scholar] |
| 9. | Bashir G, Thokar MA, Ahmad S, Fomda BA, Lone R, Fazili T. Fungemia caused by Verticillium species in an immunocompromised child. Indian J Med Microbiol 2006;24:65–6. [Google Scholar] |
| 10. | Badreddine S, Al Amoudi A, AbdelRauf B, Al-Katheeri A, Almutawa A, Al-Ghamdi SM, et al. Verticillium species skin infection in a renal transplant patient. Exp Clin Transplant 2017;1:105–7. [Google Scholar] |
Fulltext Views
2,573
PDF downloads
1,492




