Translate this page into:
Kinetics of Covid-19 antibodies in terms of titre and duration among healthcare workers: A longitudinal study
[To cite: Goenka MK, Goenka U, Patil VU, Das SS, Afzalpurkar S, Jajodia S, et al. Kinetics of Covid-19 antibody in terms of titre and duration among healthcare workers: A longitudinal study. Natl Med J India 2022;35:201–5.]
Abstract
Background
Most individuals with Covid-19 infection develop antibodies specific to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the dynamics of these antibodies is variable and not well-studied. We aimed to determine the titres of naturally acquired antibodies over a 12-week follow-up.
Methods
We recruited healthcare workers who had tested positive on a specific quantitative reverse transcription-polymerase chain reaction (qRT-PCR) for SARS-CoV-2, and then tested for the presence of immunoglobulin G (IgG) antibody against the same virus at baseline and again at 6 and 12 weeks. The antibody titre was determined by a semi-quantitative assay based on signal/cut-off ratio. Healthcare workers with antibody positivity were divided into those with high titre (ratio ≥12) and low titre (<12). Their demographic details and risk factors were surveyed through a Google form and analysed in relation to the antibody titres at three time-points.
Results
Of the 286 healthcare workers, 10.48% had high antibody titres. Healthcare workers who had tested positive by qRT-PCR and those who had received the Bacille Calmette–Guérin (BCG) vaccination or other immune-boosters had a higher frequency of high antibody titres. While there was a significant decline in antibody titres at 6 and 12 weeks, 87.46% of individuals positive for IgG antibody persisted to have the antibody even at 12 weeks.
Conclusion
Healthcare workers who tested positive for SARS-CoV-2 on qRT-PCR had a high positivity for the specific antibody, which continued to express in them even at 12 weeks. Further follow-up is likely to enhance our understanding of antibody kinetics following SARS-CoV-2 infection.
INTRODUCTION
The ongoing Covid-19 was declared a pandemic in March 2020. We still do not have a clear understanding regarding the immunological response of the host to this novel coronavirus. However, it is well known that antibodies including immunoglobulin G (IgG), IgM and IgA are generated in most patients in response to Covid-19 infection within 1–3 weeks.1–6 The humoral immune response may be variable in terms of magnitude and duration. The quantity of antibodies in plasma may be the determinant of its protective capability and the utility of convalescent plasma as a treatment.7 Experience with another coronavirus has shown that the humoral immune response is variable.8–11 While antibody to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome are known to persist even up to 3 years, those against human alpha and beta coronaviruses usually wane before 12 weeks.9,12–14 Data suggest that there is rapid disappearance of IgM antibody following Covid-19 infection.15 Durability of IgG antibody response to Covid-19, however, has not been evaluated well.3,4,15–19
We conducted a seroprevalence study of Covid-19 among healthcare workers (HCWs) in our hospital, and analysed those seropositive (IgG Ab) for Covid-19.20 It is believed that detectable IgG Ab imparts protective immunity from re-infection to that particular individual. However, it is unclear whether the antibody response is related to disease severity.
We aimed to determine the magnitude and durability of naturally acquired Covid-19 antibodies over a 12-week follow-up period. Our primary objective was to compare groups with high and low titres of antibodies to independent variables among Covid-19 cases. The secondary objective was to determine the relationship between various risk factors of Covid-19 infection and the antibody response.
METHODS
Study population
The study was approved by the internal ethical committee of the institute. Subjects included HCWs from our hospital who had IgG Ab against Covid-19 detected between July and September 2020.
We evaluated the level of IgG in HCWs who had tested positive for SARS-CoV-2 by qRT-PCR. These HCWs were tested either due to symptoms suggestive of Covid-19 disease or close contact with positive cases. The HCWs were then requested to come for a follow-up antibody testing at 6 weeks (±3 days) and again at 12 weeks (±3 days).
HCWs were divided into three groups based on the risk of exposure to Covid-19-positive patients:
High risk: Those who were working or had worked in a Covid-19 ward or an intensive care unit and those regularly involved in the testing or investigating a Covid-19 patient.
Intermediate risk: Those not belonging to either high- or low-risk groups, i.e. HCWs who are managing patients or performing procedures on patients not diagnosed or suspected to be having Covid-19. These included, but were not limited to, staff working in emergency, aerosol-generating facilities and outpatient services.
Low risk: Those who had no direct contact with the patients or their belongings, e.g. staff belonging to the administrative cadre, human resource department and marketing.
Covid-19 antibody testing
Antibodies to Covid-19 were tested in the plasma of participants using the enhanced chemiluminescence method (Vitros ECi, Ortho Clinical Diagnostics, New Jersey, USA) on a luminometer. Signal-to-cut-off ratio (S/Co) was used to semi-quantitatively categorize patients with antibodies to Covid-19 as high titre or low titre at a cut-off value of 12 (≥12 categorized as high titre).7,21
Profile questionnaire
All participants were also sent a questionnaire on Google forms either through a registered phone number or email address. This form collected variables that included demographic and clinical data. The form had 26 questions with multiple-option answers requiring either single or multiple replies. Survey questions were divided into three categories: (i) demographic details of participants; (ii) details of job profile; and (iii) medical history including symptoms or diagnosis of Covid-19 (by qRT-PCR).
Statistical analysis
Participants with high antibody titre were compared with those with low antibody titre in terms of various parameters including qRT-PCR positivity, age, gender, occupation, blood group, history of smoking, Bacille Calmette–Guérin (BCG) vaccination and comorbid conditions. During 6- and 12-week follow-up testing, the number of HCWs becoming negative for IgG Ab against Covid-19 was also recorded.
The data were compiled and later analysed by the software SPSS version 22.0 (IBM Inc, Chicago, Illinois, USA). The Chi-square test was used for comparisons of antibody titres. The McNemar test and Paired t-test were applied to compare baseline and follow-up antibody titres. Binary logistic regression was used to determine the strength of predictors. For all tests, confidence interval and p value were set at 95% and <0.05, respectively.
RESULTS
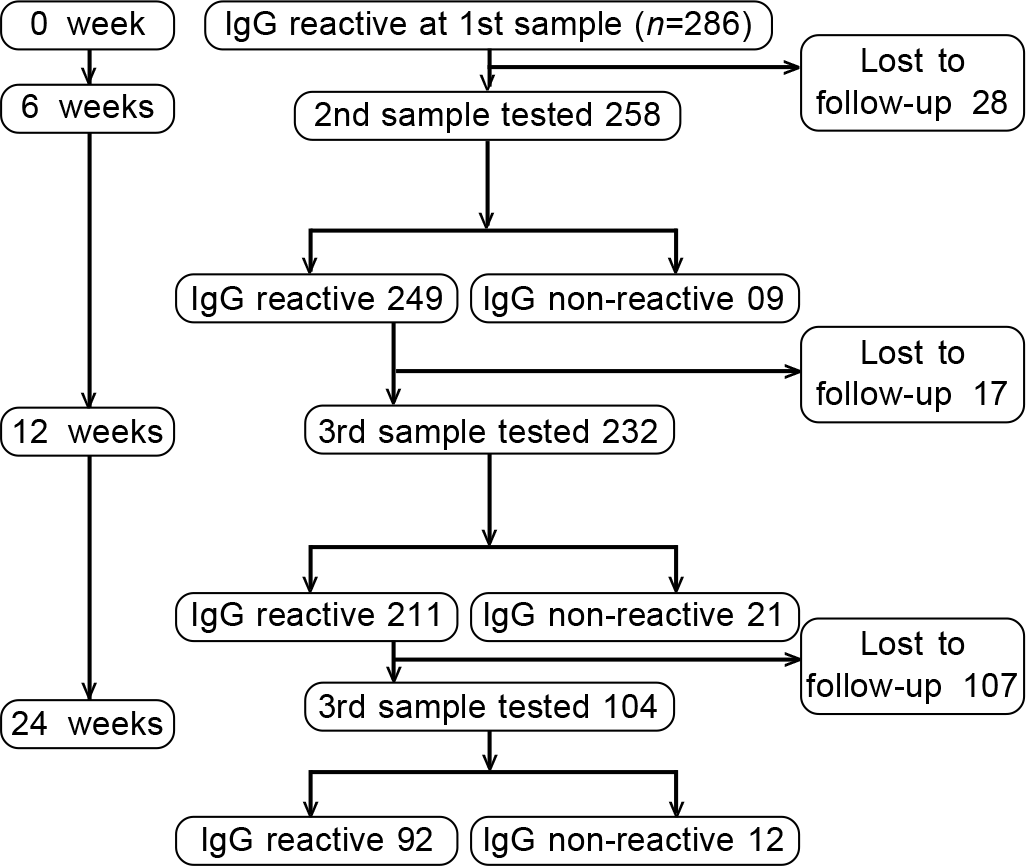
We recruited 286 HCWs who tested positive for SARS-CoV-2 IgG Ab. These included 129, who had tested positive during our seroprevalence study among HCWs but had never been positive by qRT-PCR for SARS-CoV-2. The other 157 HCWs had tested positive for antibodies 2–3 weeks after having been qRT-PCR-positive for SARS-CoV-2. Five HCWs who were qRT-PCR-positive did not have any detectable IgG SARS-CoV-2 antibodies even on sequential testing and hence were excluded from the study (Fig. 1).
- Study flowchart and result in the form of IgG Covid-19 antibody positivity
Table I compares the various parameters in these two groups at baseline.
| Independent variable | Antibody titres | p value | Total, n(%) | |
|---|---|---|---|---|
| Low (<12), n (%) | High (≥12), n (%) | |||
| qRT-polymerase chain reaction | ||||
| Positive | 128 (81.5) | 29 (18.5) | 0.001* | 157 (54.9) |
| Not positive | 128 (99.2) | 1 (0.8) | 129 (45.1) | |
| Age group (years) | ||||
| <30 | 95 (96) | 4 (4) | 0.058 | 99 (34.6) |
| 31–40 | 113 (86.9) | 17 (13.1) | 130 (45.5) | |
| 41–50 | 41 (85.4) | 7 (14.6) | 48 (16.8) | |
| 51–60 | 7 (77.8) | 2 (22.2) | 9 (3.1) | |
| Gender | ||||
| Men | 86 (90.5) | 9 (9.5) | 0.693 | 95 (33.2) |
| Women | 170 (89) | 21 (11) | 191 (66.8) | |
| Occupation | ||||
| Administration | 10 (83.3) | 2 (16.7) | 0.217 | 12 (4.2) |
| Dietician | 12 (85.7) | 2 (14.3) | 14 (4.9) | |
| Consultant doctor | 3 (60) | 2 (40) | 5 (1.7) | |
| Non-consultant doctor | 16 (80) | 4 (20) | 20 (7) | |
| Front office staff | 8 (80) | 2 (20) | 10 (3.5) | |
| Housekeeping | 74 (94.9) | 4 (5.1) | 78 (27.3) | |
| Laboratory assistant/pharmacist | 21 (84) | 4 (16) | 25 (8.7) | |
| Nurse | 64 (90.1) | 7 (9.9) | 71 (24.8) | |
| Technician | 25 (92.6) | 2 (7.4) | 27 (9.4) | |
| Ward executives | 12 (92.3) | 1 (7.7) | 13 (4.5) | |
| Others | 11 (100) | 0 | 11 (3.8) | |
| Blood group | ||||
| A | 47 (92.2) | 4 (7.8) | 0.496 | 51 (17.8) |
| Non-A | 209 (88.9) | 26 (11.1) | 235 (82.2) | |
| Smoking | ||||
| No | 202 (89.8) | 23 (10.2) | 0.777 | 225 (78.7) |
| Yes | 54 (88.5) | 7 (11.5) | 61 (21.3) | |
| Diet | ||||
| Non-vegetarian | 249 (89.2) | 30 (10.8) | 0.359 | 279 (97.6) |
| Vegetarian | 7 (100) | 0 | 7 (2.4) | |
| Bacillus Calmette–Guerin vaccination | ||||
| No | 154 (92.8) | 12 (7.2) | 0.034* | 166 (58) |
| Yes | 102 (85) | 18 (15) | 120 (42) | |
| Comorbid conditions | ||||
| Absent | 221 (90.9) | 22 (9.1) | 0.06 | 243 (85) |
| Present | 35 (81.4) | 8 (18.6) | 43 (15) | |
| Use of immune boosters | ||||
| No | 134 (93.7) | 9 (6.3) | 0.021* | 143 (50) |
| Yes | 122 (85.3) | 21 (14.7) | 143 (50) | |
| Allergic disorders | ||||
| Yes | 230 (90.2) | 25 (9.8) | 0.278 | 31 (10.8) |
| No | 26 (83.9) | 5 (16.1) | 255 (89.2) | |
| Total | 256 (89.5) | 30 (10.5) | 286 (100) | |
There was a greater probability of high titre in those who had earlier received BCG vaccination than those who had not (15.0% v. 7.2%, p=0.034). Similarly, individuals receiving immune boosters had 14.7% incidence of high titre compared to 6.3% without a history of intake of immune boosters (p=0.021). qRTPCR-positive HCWs had 24.26% times higher odds of high antibody titre than those not testing positive (p=0.002, Table II).
| Independent variable | Odds ratio | Confidence interval | p value |
|---|---|---|---|
| Polymerase chain reaction test | |||
| Positive | 24.26 | 3.227–182.467 | 0.002 |
| Negative* | |||
| Bacillus Calmette–Guerin vaccination | |||
| Received | 2.28 | 1.008–5.136 | 0.048 |
| Not received* | |||
| Use of immune boosters | |||
| Yes | 2.27 | 0.971–5.326 | 0.059 |
| No* | |||
Thirty-seven (23.57%) Covid-positive patients required hospitalization, and among them only 4 (10.8%) had high antibody titres (Table III). Low oxygen/acute respiratory distress syndrome (ARDS), need for hospitalization, duration of hospitalization and requirement of treatment with steroids and/ or antiviral drugs did not have any significant association with antibody titre (Table III).
| Independent variable | Antibody titres, n (%) | p value | Total n (%) | |
|---|---|---|---|---|
| Low, n (%) | High, n (%) | |||
| Low oxygen or acute respiratory distress syndrome | ||||
| No | 122 (80.8) | 29 (19.2) | 0.234 | 151 (96.2) |
| Yes | 6 (100) | 0 | 6 (3.8) | |
| Hospitalization | ||||
| No | 95 (79.2) | 25 (20.8) | 0.17 | 120 (76.4) |
| Yes | 33 (89.2) | 4 (10.8) | 37 (23.6) | |
| Duration of hospitalization (weeks) | ||||
| <1 | 59 (75.6) | 19 (24.3) | 0.3 | 78 (49.7) |
| 1–2 | 40 (85.1) | 7 (14.9) | 47 (29.9) | |
| 2–3 | 19 (86.4) | 3 (13.6) | 22 (14) | |
| >4 | 10 (100) | 0 | 10 (6.4) | |
| Treatment given | ||||
| No | 61 (74.4) | 21 (25.6) | 0.074 | 82 (60.3) |
| Yes | 47 (87) | 7 (13) | 54 (39.7) | |
Follow-up at 6 and 12 weeks
Of the 286 participants, 28 dropped out of the study at 6 weeks and a further 17 dropped out at 3 months (Fig. 2). Thus, 258 HCWs were tested for IgG Ab against Covid-19 at 6 weeks, while 232 HCWs were re-tested at 12 weeks. Only 9 of 258 (3.49%) become negative for antibody at 6-week follow-up, and 9.05% (21 of 232) become negative for antibody at 12 weeks. Interestingly, the 30 individuals, who became undetectable for the antibody during follow-up belonged to the low-titre group.

- Comparative assessment of high and low titres of antibodies according to Covid-19 positivity
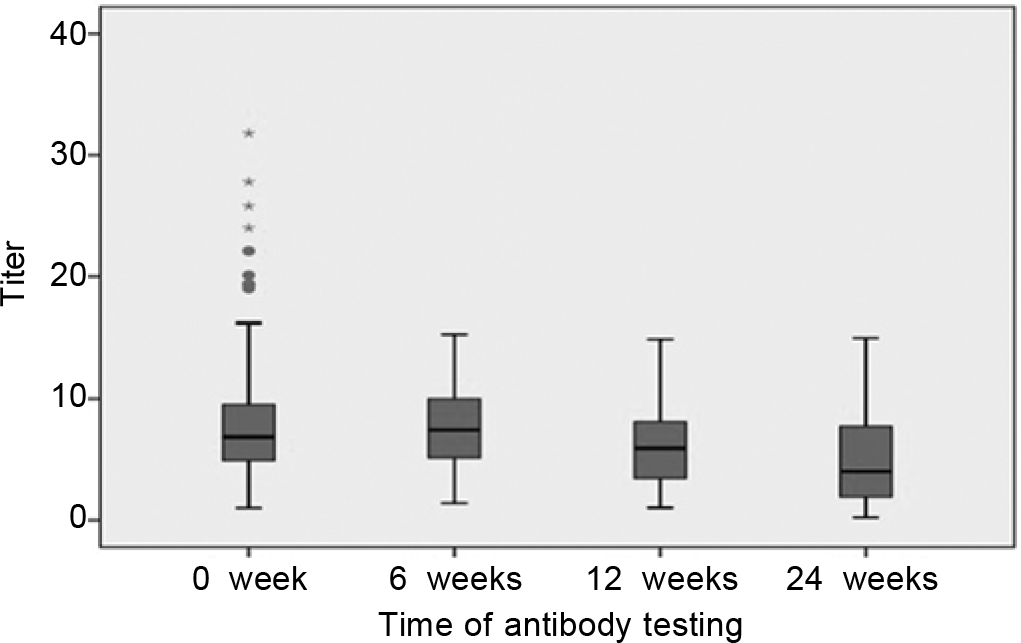
Among 232 patients evaluated at 12 weeks, the titres at 0 week, 6 weeks and 12 weeks are given in Table IV and shown in Fig. 3. The decrease in titre between the three time-periods was significant (p=0.0001, Wilcoxon signed Rank test). The symptomatic group had significantly high antibody titre not only at baseline but also on 6-week and 12-week follow-up (Table V).
- Follow-up Covid-19 IgG titres at 0, 6 and 12 weeks
| Statistic | 0 week | 6 weeks | 12 weeks |
|---|---|---|---|
| Mean (SD) | 7.80 (5.83) | 7.02 (3.85) | 5.73 (3.93) |
| Median (range) | 6.78 (1–31.8) | 6.82 (1.06–19.10) | 5.04 (0.27–17.80) |
| Variance | 34.00 | 14.82 | 15.48 |
SD standard deviation
| Time | Number of patients | Symptomatic | Asymptomatic | p value | Test |
|---|---|---|---|---|---|
| 0 weeks | Number of patients | 111 | 23 | ||
| Mean (SD) | 10.59 (7.01) | 8.04 (6.40) | <0.035 | Mann–Whitney U | |
| Median (range) | 9.06 (1.13–31.80) | 7.44 (1.60–29.20) | |||
| 6 weeks | Number of patients | 111 | 23 | ||
| Mean (SD) | 8.59 (3.63) | 5.65 (2.82) | <0.0001 | Independent samples t-test | |
| Median (range) | 8.77 (1.02–19.10) | 5.16 (1.11–11.00) | |||
| 12 weeks | Number of patients | 9 9 | 19 | ||
| Mean (SD) | 7.56 (3.58) | 5.04 (2.99) | <0.004 | Mann–Whitney U | |
| Median (range) | 7.10 (1.81–15.90) | 4.45 (1.03–11.80) |
Antibody response among the seropositive group (after excluding RT-PCR-positive patients) was compared at 6 weeks and 12 weeks from the baseline (Table VI). There was no significant drop in the mean antibody levels during follow-up after 6 weeks (p=0.9, Wilcoxon signed ranks test). However, there was a significant drop in antibody titre in the third time-period, i.e. 12 weeks in comparison to the first titre (p=0.05). Similarly, there was a drop in mean antibody levels after 12 weeks in comparison to the levels after 6 weeks (p<0.0001).
DISCUSSION
We have shown that patients with PCR positivity had a higher chance of a high antibody titre. A study by Long et al.22 from China has also shown that IgG Ab level in symptomatic Covid-19 patients was much higher compared to that in the asymptomatic group. However, high titre in our study did not correlate with severity of disease as evidenced by need for target therapy, oxygen and duration of hospitalization. This is somewhat different from the experience by Seow et al.16 who did notice a relationship between antibody titre and severity of disease. The reason for low antibodies among seropositive cases could be a reflection of the time duration between exposure and time of testing.
Our study also shows a higher antibody response in patients who had received BCG vaccination in childhood and ‘immune-boosters’. Sharma et al.23 had reported the mortality to be lower and recovery rate to be higher in countries that have BCG vaccination in their universal health programme. Malik et al.24 explained the role of BCG vaccination and its immunological effects. A few studies have evaluated the immune-boosting role of vitamins such as D, C, E, zinc, selenium and omega-3 fatty acids in the prevention and treatment of Covid-19 by improving immunity, in general.25,26 More work, however, is needed to establish these relationships.
Our study shows that 87.46% of subjects with IgG Ab against Covid-19 continue to have antibodies at 12 weeks. However, as shown in earlier studies from the UK16 and China,22 the antibody titre decreased during follow-up. The decrease or disappearance was more likely in those who had low initial antibody titre both in our data and earlier reported series. Seow et al.16 noted that individuals with high peak ID50 (serum dilution that inhibits 50% infection) for neutralization maintained high neutralizing antibody titre for longer period. Ibarrondo et al.18 and Bruni et al.19 have shown rapid decaying of anti-SARS-CoV-2 antibodies in persons with mild Covid-19.
We used Vitros anti-SARS-COV-2 IgG assay, which targets the S1 spike protein.27,28 Compared to other coronaviruses, S1 protein is more specific and unique to SARS-CoV-2.29,30 Chemiluminescence-immunoassay used in our study has been shown to be superior to the ELISA method.31
Our limitations include the modest sample size and not measuring the exact quantity of the specific IgG antibody in plasma.
Conclusion
Our study shows that IgG Ab to SARS-CoV-2 was higher in HCWs who had tested positive for SARS-CoV-2 by qRT-PCR than in those in whom antibodies were detected during the seroprevalence study. Moreover, HCWs having received BCG vaccination and administered immune boosters had higher antibody titres. We also noted that most participants continued to have antibodies even at 12 weeks, though the titre showed a significant decline. Further follow-up is needed to clearly illustrate the kinetics of antibody response in Covid-19.
ACKNOWLEDGEMENT
We thank Mr Saurav Barman for helping in the compilation of data.
Conflicts of interest
None declared
References
- A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033-6.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirusassociated illnesses. J Med Virol. 2020;92:512-17.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845-8.
- [CrossRef] [PubMed] [Google Scholar]
- Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26:1478-88.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathol. 2020;16:e1008817.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-sectional evaluation of humoral responses against SARS-CoV-2 Spike. Cell Rep Med. 2020;1:100126.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv 2020
- [CrossRef] [Google Scholar]
- The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435-46.
- [CrossRef] [PubMed] [Google Scholar]
- The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101:791-7.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal profile of antibodies against SARScoronavirus in SARS patients and their clinical significance. Respirology. 2006;11:49-53.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 vaccines: 'Warp Speed' needs mind melds not warped minds. J Virol. 2020;94:1-32.
- [CrossRef] [PubMed] [Google Scholar]
- Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691-3.
- [CrossRef] [PubMed] [Google Scholar]
- Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162-3.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal analysis of severe acute respiratory syndrome (SARS) coronavirus-specific antibody in SARS patients. Clin Diagn Lab Immunol. 2005;12:1455-7.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. J Infect Dis. 2020;222:183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598-607.
- [CrossRef] [PubMed] [Google Scholar]
- Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020
- [CrossRef] [Google Scholar]
- Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085-7.
- [CrossRef] [PubMed] [Google Scholar]
- Persistence of anti-SARS-CoV-2 antibodies in non-hospitalized COVID-19 convalescent health care workers. J Clin Med. 2020;9:3188.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68:38-43.
- [CrossRef] [Google Scholar]
- U.S. Department of Health and Human Services. Available at www.fda.gov/media/141477 (accessed on 1 Feb 21)
- [Google Scholar]
- Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200-4.
- [CrossRef] [PubMed] [Google Scholar]
- BCG as a game-changer to prevent the infection and severity of COVID-19 pandemic? Allergol Immunopathol (Madr). 2020;48:507-17.
- [CrossRef] [PubMed] [Google Scholar]
- BCG vaccine: A hope to control COVID-19 pandemic amid crisis. Hum Vaccin Immunother. 2020;16:2954-62.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition. 2020;79-80:110948.
- [CrossRef] [PubMed] [Google Scholar]
- Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58:e01243-20.
- [CrossRef] [PubMed] [Google Scholar]
- Testing for SARS-CoV-2: The day the world turned its attention to the clinical laboratory. Clin Transl Sci. 2020;13:871-6.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58:e00461-20.
- [CrossRef] [PubMed] [Google Scholar]
- Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478-88.
- [CrossRef] [PubMed] [Google Scholar]




