Translate this page into:
MICHELLE trial: Inching towards the holy grail of post-discharge anticoagulation in Covid-19!
Ramacciotti E, Agati LB, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, dos Santos JL, Volpiani GG, Sobreira ML, Joviliano EE, Júnior MSB, da Fonseca BAL, Ribeiro MS, Dusilek C, Itinose K, Sanches SMV, de Almeida Araujo Ramos K, de Moraes NF, Tierno PFGMM, de Oliveira ALML, Tachibana A, Chate RC, Santos MVB, de Menezes Cavalcante BB, Moreira RCR, Chang C, Tafur A, Fareed J, Lopes RD, on behalf of the MICHELLE investigators. (Science Valley Research Institute, São Paulo, Brazil; Hospital e Maternidade Christóvão da Gama, Grupo Leforte, Santo André, São Paulo, Brazil; Unidade de Medicina Interdisciplinarem Cardiologia, Instituto do Coração, Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil; Zucker School of Medicine at Hofstra/Northwell and the Feinstein Institutes for Medical Research, Manhasset, NY, USA; Department of Obstetrics and Gynecology, I.M. Sechenov First Moscow State Medical University, Moscow, Russia; Universidade Estadual Paulista, Botucatu, Brazil; Hospital das Clínicas de Ribeirão Preto, São Paulo University Medical School, Ribeirão Preto, São Paulo, Brazil; Hospital do Rocio, Campo Largo, Paraná, Brazil; Instituto Couto Maia, Salvador, Bahia, Brazil; Hospital Municipal de Barueri, São Paulo, Brazil; São Paulo State Public Women’s Health Reference Center, São Paulo, Brazil; Hospital Israelita Albert Einstein, São Paulo, Brazil; Instituto do Coração, Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil; Institute of Teaching and Research Hapvida, Fortaleza, CE, Brazil; Hospital Nossa Senhora das Graças, Curitiba, Brazil; Department of Statistics, Institute of Mathematics and Statistics, University of São Paulo, São Paulo, Brazil; Northshore University Health System, Chicago, IL, USA; Hemostasis and Thrombosis Research Laboratories at Loyola University Medical Center, Maywood, IL, USA; Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA.) Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalization for COVID-19 (MICHELLE): An open-label, multicenter, randomized, controlled trial. Lancet 2022;399:50–9.
SUMMARY
The MICHELLE study was an open-label, multicentre, randomized, controlled trial to assess the role of extended thromboprophylaxis (with oral anticoagulation) after hospitalization with Coronavirus disease 2019 (Covid-19) in lowering the venous or arterial thromboembolic events. Patients hospitalized with Covid-19 were included in the trial after discharge from hospital if they were at increased risk of thromboembolic events. Increased venous thromboembolic risk was ascertained by an elevated modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE score of 2–3 with a D-dimer >500 ng/ml or a score of >4. A total of 320 such patients were randomized at discharge to rivaroxaban 10 mg/day or no anticoagulation in a 1:1 ratio for 35 days. Patients with a known thrombotic event were not included in the study. About 52% of patients required care in intensive care unit (ICU) during hospital stay and the mean duration of hospitalization was 8 days (range 6–12 days). A total of 86% of patients received thromboprophylaxis with enoxaparin 40 mg once a day during hospital stay. Sixty-two per cent patients had an IMPROVE VTE score of 2–3 along with increased D-dimer levels while overall 92% had increased D-dimer levels (above the upper limit of normal of 500 ng/ml). At day 35 post-discharge, venous thromboembolic events were recorded by bilateral lower limb venous doppler ultrasound and computed tomography (CT) pulmonary angiograms. There was a significant reduction in primary efficacy outcome in the rivaroxaban group v. placebo (3.14% in the rivaroxaban arm v. 9.43% in the control group) with a relative risk (RR) reduction of 67% (p=0.0293). This included a composite of symptomatic or fatal venous thromboembolism (VTE), asymptomatic VTE detected by bilateral lower limb venous doppler ultrasound or CT pulmonary angiogram, symptomatic arterial thromboembolism (MI, non-haemorrhagic stroke and major adverse limb event) and cardiovascular (CV) death at day 35. This primary outcome was mainly influenced by increased events of pulmonary thromboembolism in the control group. There was one symptomatic and one asymptomatic pulmonary embolism event in the treatment group while there were four asymptomatic, three symptomatic, and three fatal pulmonary embolism events in the control group.
The incidence of symptomatic and fatal VTE events was significantly reduced in the rivaroxaban group to 1 patient, while it was reported in 8 patients in the control group (RR 0.13, 95% CI 0.02–0.99, p=0.0487). The composite of venous and arterial thromboembolism and CV death was also significantly lesser in the rivaroxaban group, with a total of such events occurring in 1 patient in the rivaroxaban group and in 9 patients in the control group (RR 0.11, 95% CI 0.01–0.98, p=0.036). There was no event of International Society on Thrombosis and Haemostasis (ISTH) defined major bleeding in either group. However, clinically relevant non-major bleeding occurred in 2 patients each in the rivaroxaban group and the control group. To conclude, extended thromboprophylaxis with oral anticoagulant in patients hospitalized with Covid-19 and at higher thrombotic risk yields favourable results. Contrary to the results of previous trials, there was a greater benefit perhaps due to the enrolment of only those patients with higher thrombotic risk. There was also no significant increase in major bleeding events in the rivaroxaban arm, perhaps due to the exclusion of patients who had active cancer or gastrointestinal ulcer, bronchiectasis, bleeding in the previous 3 months, recent surgery, use of dual antiplatelet therapy, creatinine clearance below 30 ml/minute or those receiving dual antiplatelet therapy.
COMMENT
Covid-19 presents as a multisystemic disease with inflammation playing a major role in the involvement of various organs. There is an exacerbated immune response that leads to tissue damage and multi-organ dysfunction. Due to the hyperimmune response, there is endothelial dysfunction, macrophage activation syndrome and increased risk of thrombosis.1 An increase in coagulation factors (II, VII, IX, X), von Willebrand factor and antiphospholipid antibodies have also been noted, all contributing to thrombosis.2 There is increased risk of disseminated intravascular coagulation (DIC) and bleeding.1
Cytokine release leading to acute respiratory distress syndrome has been a major cause of mortality in patients with Covid-19. Pulmonary embolism has been the second most common cause of death in such patients.3 Among patients with severe Covid-19 admitted to ICU, there has been an incidence of symptomatic VTE of almost 25%.4 There are yet no clear guidelines regarding the indications and choice of anticoagulants in SARS-CoV-2 infection. According to the consensus, patients with severe illness are candidates for prophylactic or therapeutic doses of parenteral anticoagulation.5,6 Benefits of anticoagulation for hospitalized patients are well documented.7 Non-adherence (missing >2 days dose) to anticoagulation has been linked to higher 60-day mortality (adjusted HR 1.31, 95% CI 1.03–1.67), while receiving any anticoagulation versus none led to lower inhospital mortality (adjusted HR 0.38, 95% CI 0.25–0.58). The choice of therapeutic versus prophylactic anticoagulation is contentious. While the HEP-COVID trial clearlyproved benefits of therapeutic dose low molecular weight heparin (LMWH) in reducing death and major thromboembolic events, the RAPID trial failed to sustain the benefits of therapeutic dose heparin.8,9 The REMAP-CAP, ACTIV-4a, and ATTACC investigators did not find merit in therapeutic dose anticoagulation with heparin for critically ill patients while in non-critically ill patients it improved probability of survival and organ support-free days.10,11 There is an impression that in advanced stages of Covid-19 the changes become irreversible precluding benefits of heparin. Thus, a large body of data still favour a prophylactic strategy of heparin/LMWH in hospitalized patients. On the contrary, episodes of thromboembolism despite prophylactic doses of anticoagulants may argue towards therapeutic anticoagulation in hospitalized patients.12 Concerns regarding increased episodes of bleeding may warrant against their use in non-critically ill outpatients. Of course, Covid-19 patients with radiographically/imaging diagnosed thrombotic events are candidates for therapeutic anticoagulation for at least 3 months unless they are critically ill.
Despite the general consensus on parenteral anticoagulation (prophylactic dose heparin) for hospitalized patients for Covid-19, there is dearth of evidence for post-discharge anticoagulation in the absence of proven arterial thromboembolism or VTE. Both the type of anticoagulation (oral/parenteral) and the duration of therapy were subjects of additional research.
The MAGELLAN trial (n=8101) has shown that extended duration prophylaxis with rivaroxaban 10 mg (following discharge for 31–39 days) was non-inferior to enoxaparin (6–14 days) for prevention of thromboembolic events in acute medically ill patients admitted to ICU.13 Asymptomatic proximal deep vein thrombosis, symptomatic proximal or distal deep vein thrombosis, symptomatic non-fatal pulmonary embolism and death related to VTE were the four components of the composite primary outcome studied. In fact, by day 35 the drug was superior to enoxaparin. However, bleeding events were also higher with extended prophylaxis.
The MARINER trial (n=12 019) used Modified IMPROVE VTE score and D-dimer levels to enrol patients for extended prophylaxis.14 Symptomatic VTE and/or death due to VTE were the composite primary end-points. Although the trial failed to show reduction in the primary end-point, symptomatic non-fatal VTE was reduced by 56%. There was no increase in bleeding with extended prophylaxis. In a subsequent analysis, extended duration (45 days) post-discharge rivaroxaban 10 mg daily for hospitalized medically ill patients resulted in a significant 28% reduction in fatal and major thromboembolic events without causing a significant increase in major bleeding events.15 It also resulted in a RR reduction of 80% and 62% in symptomatic lower limb deep vein thrombosis and symptomatic non-fatal pulmonary embolism, respectively. There was no statistically significant increase in major bleeding events. The use of clinical and biochemical parameters to triage patients at higher thromboembolic risk could have led to lower bleeding in the MARINER trial compared to blanket enrolment in the MAGELLAN trial. Hence, it is crucial to avoid events in this prolonged phase of increased thrombosis. The drug is approved by the US Food and Drug Administration for post-ICU extended VTE prophylaxis in patients not at high risk of bleeding. This is more appropriate for Covid-19 as it has a high rate of arterial thromboembolism and VTE.15 However, specific trials in the setting of Covid-19 were lacking.
The largest registry enrolling 4906 Covid-19 patients prospectively elucidated that there are definite episodes of arterial and venous thrombosis in patients even after discharge from hospital.16 This risk was reduced by 46% in those receiving prophylactic post-discharge anticoagulation. Interestingly, only 13% were prescribed oral anticoagulation at discharge in the registry.
The ACTIV-4B trial also evaluated oral anticoagulation in stable outpatients with Covid-19 across 52 centres in the USA.17 Patients were randomized to aspirin and two different doses of apixaban (2.5 mg and 5 mg twice a day). Both ischaemic and bleeding outcomes were evaluated. The composite of all-cause mortality, symptomatic VTE or arterial thromboembolism, myocardial infarction, stroke or hospitalization for cardiovascular or pulmonary cause was not lowered either with aspirin or apixaban. Though, the trial was prematurely terminated, bleeding events were higher with antithrombotic drugs.
Appropriate dosing of anticoagulant is vital as the ACTION trial showed an increase in bleeding with therapeutic dose 20 mg rivaroxaban being used for in-hospital and up to 30 days post-discharge Covid-19 patients with elevated D-dimer levels.18 This trial did not report any considerable advantage of extended therapeutic anticoagulation in terms of VTE, myocardial infarction, stroke, systemic embolism or major adverse limb events.
| VTE risk factor | Score |
|---|---|
| Previous venous thromboembolism (VTE) | 3 |
| Known thrombophilia | 2 |
| Current lower limb paralysis or paresis | 2 |
| History of cancer | 2 |
| Intensive care unit/coronary care unit stay | 1 |
| Complete immobilization >1 day | 1 |
| Age >60 years | 1 |
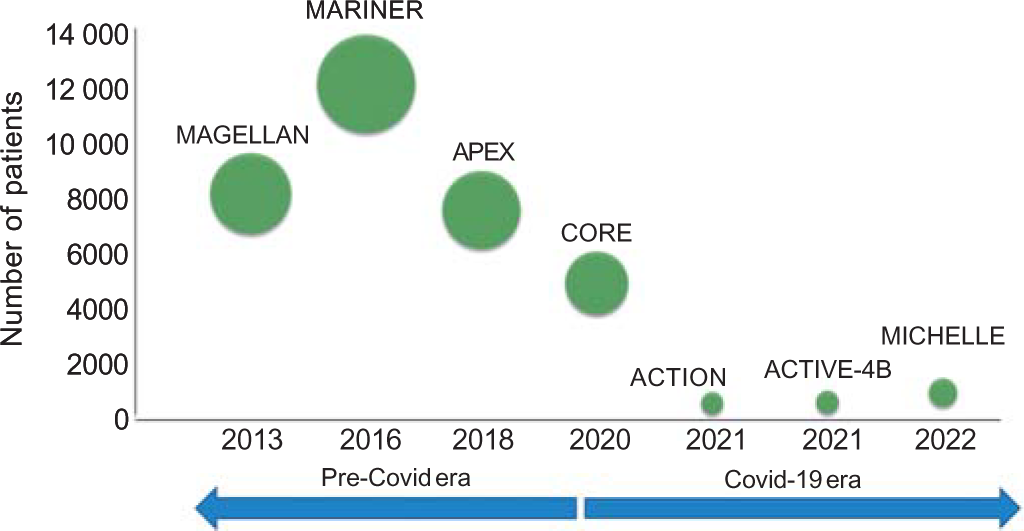
- Various studies for post-discharge extended thromboprophylaxis with oral anticoagulants in the past decade. See text for details of individual studies. The size of a circle is proportional to the number of patients enrolled in that study
These results differ from the MICHELLE trial as routine screening for thrombotic events was done in the latter by lower limb venous doppler and pulmonary CT angiography. This helped pick the missed thrombotic episodes. The asymptomatic thromboembolic events cannot be neglected as earlier data have shown all-cause mortality to be two times higher among those with asymptomatic proximal deep vein thrombosis.19 This screening of all asymptomatic patients also led to an additional 11% relative risk reduction in thromboembolic events in the MICHELLE trial over the MARINER trial. Additionally, use of Modified IMPROVE VTE and D-dimer led to the selection of a cohort poised clinically and biochemically at high thrombotic risk in which the risk of bleeding is counterbalanced leading to net clinical benefits as seen in the MARINER trial. The rather blanket/all comer enrolment followed in the MEGELLAN and ACTIV-4B trials probably led to dilution of ischaemic benefits with bleeding events.
Rivaroxaban is now off-patent and its cost has come down dramatically. This augurs well from a public health perspective as most of health expenditure is out-of-pocket due to poor insurance penetration in India.
Results of the MICHELLE trial for post-discharge thromboprophylaxis cannot be extrapolated to all outpatient management of stable Covid-19 patients. It is the continuing thrombotic risk for a prolonged duration in hospitalized patients with Covid-19 which needs attention. There was shown to be no added advantage with either aspirin or anticoagulation in symptomatic but stable outpatients with Covid-19.18
Research is ongoing in this field. The ACTIV-4c trial (NCT04650087) is designed to study the effectiveness and safety of three agents—apixaban, aspirin and placebo—in moderate-to-severe Covid-19 patients after discharge from the hospital. The primary objective is to assess myocardial infarction, stroke, arterial and venous thrombosis, and death within 30 days after hospital discharge in such patients. Another multicentre, randomized study, XACT trial (NCT04640181) aims to study the potential benefits of LMWH during hospitalization v. rivaroxaban 10 mg daily during hospital stay and continued after discharge for a total of 28 days. The CARE trial (NCT0475785) and APOLLO trial (NCT04746339) are evaluating rivaroxaban and apixaban, respectively, in the outpatient setting to reduce mortality.
To conclude, it is important to understand how the MICHELLE trial and hence its results differ from the previous negative trials. The patient population was appropriately selected for inclusion based on a higher thrombotic risk. It is perhaps due to the inclusion of patients with both elevated biochemical marker (Ddimer levels) and higher clinically assessed risk of thrombosis by the IMPROVE VTE score. Also, safety results were not compromised as the patients with definite high risk of bleeding were already excluded. Such a well-selected cohort of hospitalized patients should be the ideal candidates for extended anticoagulant therapy. Rivaroxaban seems to be an attractive choice in India as well due to easy availability, reduced costs after being off-patent and once daily dosing.
References
- COVID-19 and cardiovascular diseases: Challenges and solutions. Cardiol Res. 2021;12:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- British Thoracic Society (BTS) guidance on venous thromboembolic disease in patients with COVID-19. Available at www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-guidance-on-venousthromboembolic-disease-in-patients-with-covid-19 (accessed on 20 Jan 2022)
- [Google Scholar]
- Anticoagulation for COVID-19 patients: A bird's-eyeview. Clin Appl Thromb Hemost. 2021;27:10760296211039288.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of venous thromboembolism inpatients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421-4.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859-65.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention, diagnosis, and treatment of VTE in patients with corona virus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143-63.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in venous thromboembolism anticoagulation in patients hospitalized with COVID-19. JAMA Netw Open. 2021;4:e2111788.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: The HEP-COVID randomized clinical trial In: JAMA Intern Med. Vol 181. 2021. p. :1612-20. Erratum JAMA Intern Med 2022;182:239
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with Covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777-89.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790-802.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211-16.
- [CrossRef] [PubMed] [Google Scholar]
- Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-23.
- [CrossRef] [PubMed] [Google Scholar]
- Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118-27.
- [CrossRef] [PubMed] [Google Scholar]
- Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75:3140-7.
- [CrossRef] [PubMed] [Google Scholar]
- Post discharge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: The CORE-19 registry. Blood. 2021;137:2838-47.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: The ACTIV-4B randomized clinical trial. JAMA. 2021;326:1703-12.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253-63.
- [CrossRef] [PubMed] [Google Scholar]
- Association between asymptomatic proximal deep vein thrombosis and mortality in acutely ill medical patients. J Am Heart Assoc. 2021;10:e019459.
- [CrossRef] [PubMed] [Google Scholar]




