Translate this page into:
Mitral valve repair in an octogenarian with symptomatic severe mitral regurgitation: First use of MitraClip in India
2 Apollo Heart Institute, Chennai, Tamil Nadu, India and Mayo Clinic, Rochester, USA
3 Cedars Sinai Medical Center, Los Angeles, CA, USA
Corresponding Author:
Krishnaswamy Chandrasekaran
Apollo Heart Institute, Chennai, Tamil Nadu, India and Mayo Clinic, Rochester
USA
kchandra1950@gmail.com
| How to cite this article: Satish S, Chandrasekaran K, Makar M, Mao R, Oomen A, Shankar V, Sadasivan C, Chandrasekar GA, Senthil H, Kalarickal MS, Kar S. Mitral valve repair in an octogenarian with symptomatic severe mitral regurgitation: First use of MitraClip in India. Natl Med J India 2020;33:207-209 |
Abstract
Percutaneous mitral valve repair is an accepted treatment of choice in Europe and North America for severe primary or secondary mitral regurgitation, in highly symptomatic patients for whom surgical repair is prohibitively high risk. We describe the first use of the MitraClip in India in a frail elderly female with symptomatic heart failure from severe primary mitral regurgitation who was considered high risk for surgical repair. She had substantial improvement in her symptoms as well as quality of life following the procedure.Introduction
Percutaneous edge-to-edge mitral valve interventions began in 2004 to address primary mitral valve regurgitation.[1] The safety and short-term efficacy of the MitraClip (TM Abbot Vascular) were addressed by the EVEREST phase I trial.[2] MitraClip received the initial CE Mark approval in Europe in 2008 and was approved by the Food and Drug Administration in 2013 for treating high-risk patients with primary mitral regurgitation (MR) who are not eligible for open-heart surgery. Several studies over the past decade have confirmed its safety and efficacy in reducing the MR as well as improvement in quality of life.[3],[4],[5]
We report one of the early successful percutaneous mitral valve repairs in India with MitraClip in an octogenarian with symptoms of debilitating heart failure (HF) from severe primary MR.
The Case
An 85-year-old relatively asymptomatic Indian female was hospitalized in March 2017 for sudden onset of shortness of breath. She was found to have acute HF due to severe MR caused by an unsupported segment from chordal rupture of a severely prolapsing posterior mitral leaflet; the size and function of the left ventricle (LV) were normal and the left atrium (LA) was mildly enlarged in the anteroposterior dimension, 4.8 cm. Her medical history was relevant for mild bronchial asthma and mitral valve prolapse with mild MR. The severity of MR had remained stable since 2001. Ever since this episode, there was a considerable decrease in her functional capacity affecting her quality of life. She could barely walk 100 m without having to rest. Since then, she had multiple hospitalizations for HF despite being on optimal medical therapy. A cardiothoracic surgical consult was obtained, but surgery was deferred in view of her age, history of longstanding bronchial asthma and frailty score of 6.
Between March 2017 and November 2018, she had four hospitalizations for HF and 11 visits to the outpatient department. While her LV function was preserved, her MR seemed to have worsened in the review of October 2017. Her HF medication was optimized with diuretics and ACE inhibitors. She did not tolerate even a small dose of beta blockers. Her clinical condition deteriorated progressively. She was hospitalized in January 2018 with acute HF. Coronary angiogram was normal. Since her clinical condition was deteriorating rapidly despite optimal medical management, and her surgical risk was high with a EuroScore of 8% and Society of Thoracic Surgery (STS) score of 10%, it was felt that she could benefit from a less morbid percutaneous mitral valve intervention with MitraClip. In June 2018, a trans-oesophageal echocardiogram (TEE) was performed which confirmed a flail middle segment (P2) of the posterior leaflet and severe anterior and medially directed regurgitation ([Figure - 1] and [Figure - 3], left panel), while the LA was enlarged 5.2 cm in the anteroposterior dimension, it was only 4.3 cm in the superior-inferior dimension barely meeting the cut value of >4 cm to be eligible for the MitraClip. The leaflet morphology was suitable for the clip. The flail width was 14 mm, flail gap was 12 mm and coaptation length was 5 mm. The estimated right ventricular systolic pressure was 62 mmHg. She subsequently underwent the MitraClip procedure on 28 November 2018. Initially, one clip was used, grasping the mid portion of the P2 and corresponding A2, the MR improved considerably, the mean left atrial pressure decreased [Table - 1]; however, there was residual mild regurgitation anterior to the clip, which could not be improved by fine adjustment of the first clip. Transmitral Doppler showed a mean diastolic gradient of 3 mmHg, indicating that the mitral orifice will not be compromised by deploying another clip to obtain an optimal result. Therefore, the first clip was deployed grasping the middle of P2 and A2, and another clip was deployed adjacent and anterior to the first clip resulting in trivial residual regurgitation ([Figure - 2] and [Figure - 3], right panel). There was no change in the mitral diastolic of 3 mmHg after deployment of the second clip. The LA pressure corresponding to ‘V’ wave indicating the pressure burden borne by the LA decreased from 44 mmHg to 28 mmHg after the first clip and to 12 mmHg after deployment of the second clip [Table - 1].
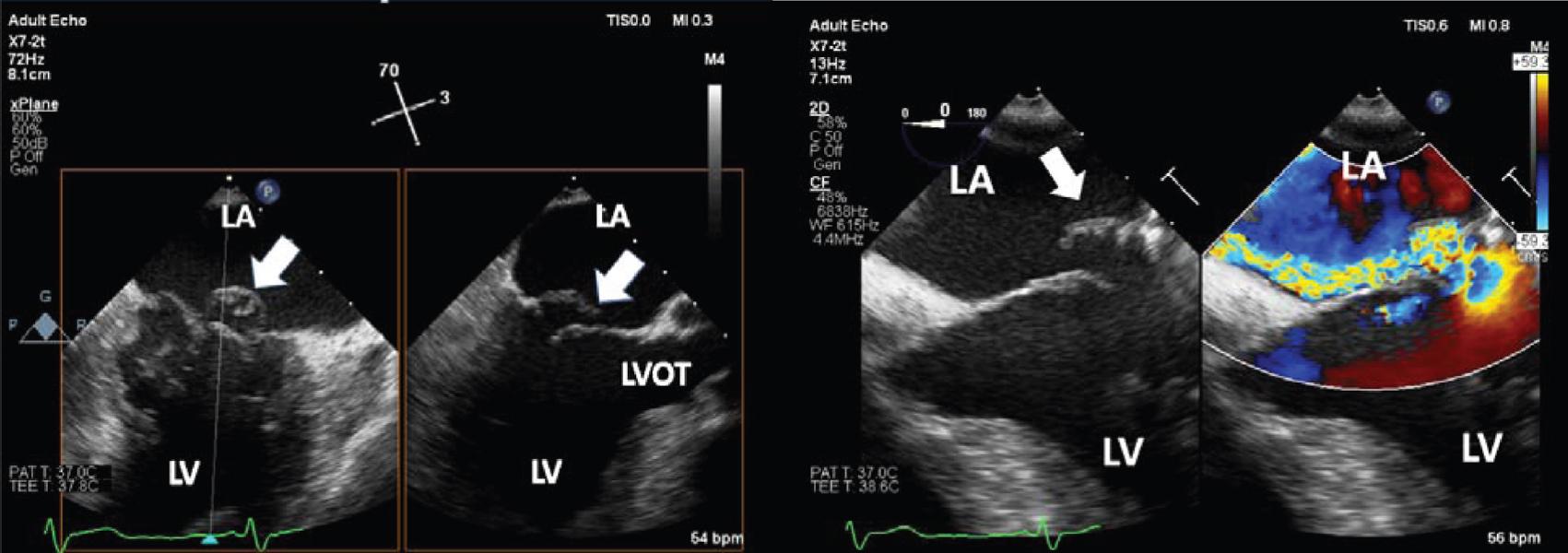 |
| Figure 1: Trans-oesophageal echocardiogram mid-esophageal 2-chamber commissural and long-axis view on the left panel and 4-chamber view with and without colour flow on the right panel demonstrates flail P2 segment of the posterior mitral leaflet with mitral regurgitation jet directed medially. Arrows point to the flail P2 segment LA left atrium LV left ventricle LVOT left ventricular outflow tract |
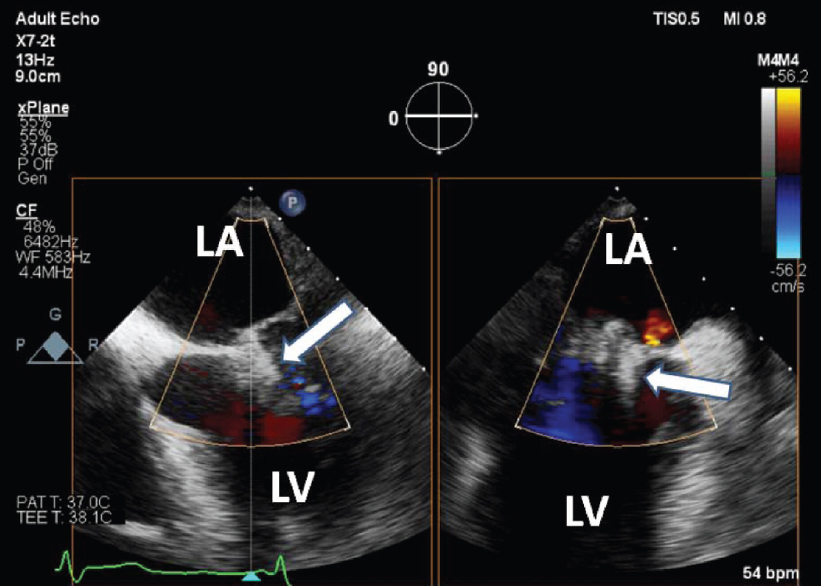 |
| Figure 2: Trans-oesophageal echocardiogram mid-oesophageal 4-chamber and 2-chamber view after the MitraClip demonstrating trivial residual mitral regurgitation. Arrows point to the MitraClip LA left atrium LV left ventricle |
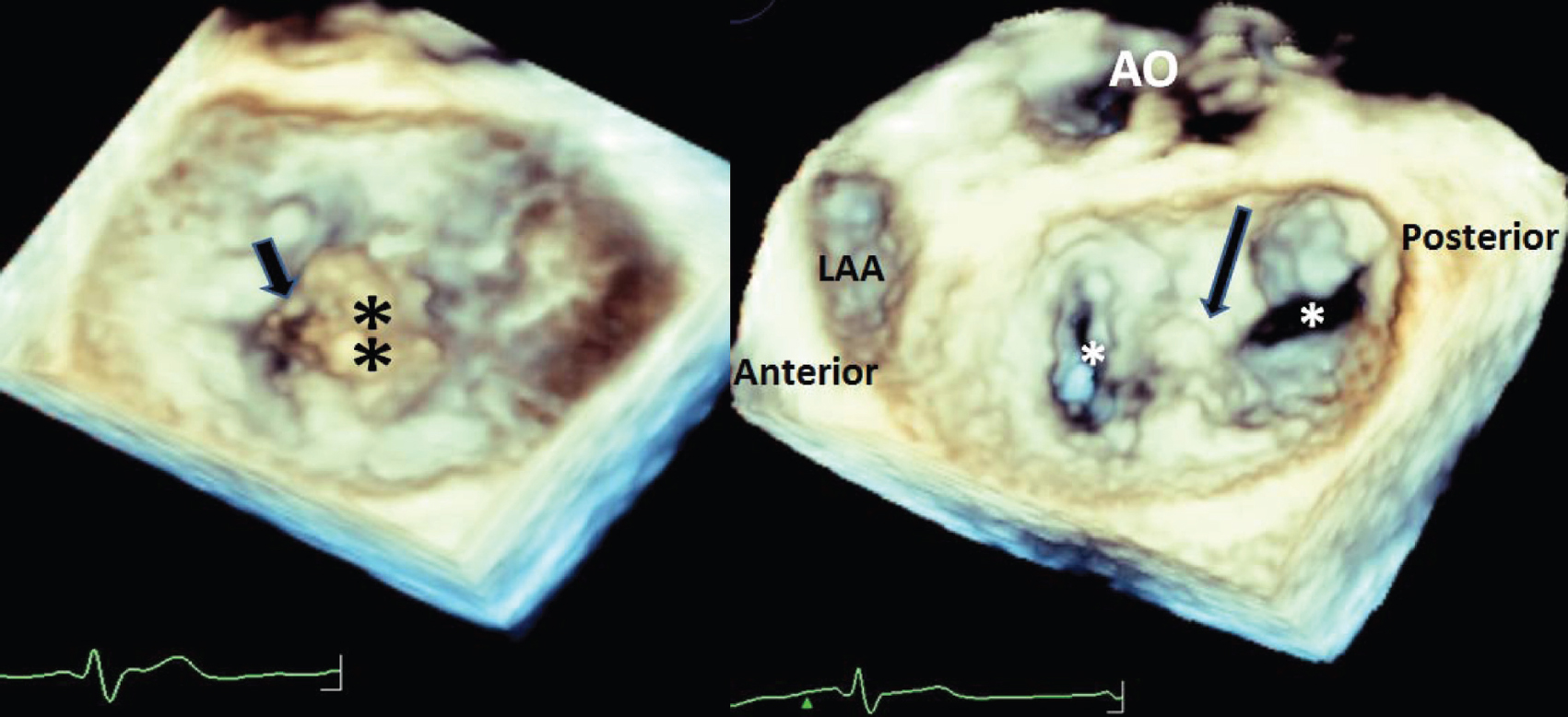 |
| Figure 3: Enface three-dimensional image of the mitral valve before the MitraClip on the left and after the MitraClip on the right panel. The flail P2 segment is denoted by the black asterisks, and the ruptured chordae is pointed by the black arrow on the left panel. The right panel after the MitraClip pointed by the black arrow demonstrates two orifices denoted by the white asterisks AO aorta LAA left atrial appendage |

She tolerated the procedure well, was extubated the following day and was discharged 2 days later. Two weeks later, her functional class had improved from NYHA class 3+ to 2. At 6 months’ follow-up, she continued to do well, had no symptoms with her daily activities (NYHA class 2) and her echocardiogram revealed normal LV function, trivial residual MR, mitral mean diastolic gradient of 2 mmHg and RV systolic pressure of 25 mmHg [Figure - 2].
Discussion
The prevalence of MR increases with age. About 10% of patients >75 years of age have symptomatic MR.[6] Non-surgical management for severe MR from flail leaflet is associated with increased adverse outcomes. Grigioni et al. have reported an incidence of 5.4% for atrial fibrillation, 8.0% for HF and 2.6% for death compared to those who underwent surgical treatment.[7] Often, elderly patients with symptomatic MR were not offered a surgical option, primarily based on prohibitive surgical risk determined by the EuroScore and STS score.[8],[9] Zhuge et al. in their study of patients >65 years of age with MR reported that of the 680 elderly patients with MR, 308 patients were denied surgery (45.29%), much higher than the rate of denial observed in the control group (45.29% v. 36.10%, p<0.001).[10] Our patient was felt to be very high risk for conventional surgical treatment based on the frailty, STS and EuroScore.
MitraClip is a grasping device delivered through the percutaneous technique. It grasps and brings together the non- coapting edges of the mitral leaflet at the site of the MR, thereby obliterating the regurgitation; converting a single large orifice into two smaller orifices. This is similar to the original mitral valve repair referred to commonly as the Alfieri stitch described by Maisano et al. in 1998.[11] The details of the procedure are described in a review by Kelley et al.[12] Our patient underwent percutaneous mitral valve repair with MitraClip successfully without any complications. She received two clips and had trace residual MR. After the first clip with residual mild regurgitation the ‘V’ wave was 28 mmHg and after the second it decreased to 12 mmHg [Figure - 3]. This shows that symptoms in acute MR depend not only on the severity of MR but also on the compliance of the LA. Mild MR in a non-compliant LA can elevate the pressure and result in symptoms. It is not uncommon to have more than one clip placed. Studies in the USA and Europe have shown over 90% procedural success, defined as post-implantation MR <Grade II and in-hospital mortality rates of 0%–4%.[13],[14] MitraClip has proven to be effective in reducing MR and improving the quality of life with survival rates comparable to surgically treated patients, despite high risk prohibiting surgical treatment.[15]
Our patient had improvement in her symptoms as well as in functional status in the early post-procedural period, which was the same till after 6 months.
Conflicts of interest. None declared
| 1. | Fann JI, St Goar FG, Komtebedde J, Oz MC, Block PC, Foster E, et al. Beating heart catheter-based edge-to-edge mitral valve procedure in a porcine model: Efficacy and healing response. Circulation 2004;110:988–93. [Google Scholar] |
| 2. | Feldman T, Wasserman HS, Herrmann HC, Gray W, Block PC, Whitlow P, et al. Percutaneous mitral valve repair using the edge-to-edge technique: Six-month results of the EVEREST phase I clinical trial. J Am Coll Cardiol 2005;46: 2134–40. [Google Scholar] |
| 3. | Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395–406. [Google Scholar] |
| 4. | Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: The EVEREST II (Endovascular valve edge-to-edge repair) high risk study. J Am Coll Cardiol 2012;59:130–9. [Google Scholar] |
| 5. | Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 2015;66:2844–54. [Google Scholar] |
| 6. | Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: A population-based study. Lancet 2006;368: 1005–11. [Google Scholar] |
| 7. | Grigioni F, Tribouilloy C, Avierinos JF, Barbieri A, Ferlito M, Trojette F, et al. Outcomes in mitral regurgitation due to flail leaflets. A multicenter European study. JACC Cardiovasc Imaging 2008;1:133–41. [Google Scholar] |
| 8. | Society of Thoracic Surgeons. Online STS Risk Calculator. Available at http:// riskcalc.sts.org/STSWeb RiskCalc273 (accessed on 10 Jan 2019) [Google Scholar] |
| 9. | Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, et al. ESC working group on valvular heart disease position paper: Assessing the risk of interventions in patients with valvular heart disease. Eur Heart J 2012;33:822–8, 828a, 828b. [Google Scholar] |
| 10. | Zhuge RQ, Hou XP, Qi XL, Wu YJ, Zhang MZ. Clinical features and treatment options for mitral regurgitation in elderly inpatients. J Geriatr Cardiol 2018;15:428–33. [Google Scholar] |
| 11. | Maisano F, Torracca L, Oppizzi M, Stefano PL, D’Addario G, La Canna G, et al. The edge-to-edge technique: A simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg 1998;13:240–5. [Google Scholar] |
| 12. | Kelley C, Lazkani M, Farah J, Pershad A. Percutaneous mitral valve repair: A new treatment for mitral regurgitation. Indian Heart J 2016;68:399–404. [Google Scholar] |
| 13. | Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, et al. Percutaneous mitral valve edge-to-edge repair: In-hospital results and 1-year follow-up of 628 patients of the 2011–2012 pilot European sentinel registry. J Am Coll Cardiol 2014;64:875–84. [Google Scholar] |
| 14. | Sorajja P, Mack M, Vemulapalli S, Holmes DR Jr, Stebbins A, Kar S, et al. Initial experience with commercial transcatheter mitral valve repair in the United States. J Am Coll Cardiol 2016;67:1129–40. [Google Scholar] |
| 15. | Swaans MJ, Bakker AL, Alipour A, Post MC, Kelder JC, de Kroon TL, et al. Survival of transcatheter mitral valve repair compared with surgical and conservative treatment in high-surgical-risk patients. JACC Cardiovasc Interv 2014;7: 875–81. [Google Scholar] |
Fulltext Views
2,188
PDF downloads
1,309




