Translate this page into:
Non-arteritic anterior ischaemic optic neuropathy and obstructive sleep apnoea
Correspondence to SIDDHARTH MADAN; drsiddharthmadan@gmail.com
[To cite: Madan S, Sethi M, Bajpai V, Garg R. Non-arteritic anterior ischaemic optic neuropathy and obstructive sleep apnoea. Natl Med J India 2023;36:364–7. DOI: 10.25259/NMJI_982_20]
Abstract
Anterior ischaemic optic neuropathies (AIONs) are a common cause of permanent visual loss in the elderly population. The non-arteritic subtype has been intensively studied. While systemic associations such as hypertension and diabetes mellitus are commonly recognized and treated, others such as obstructive sleep apnoea (OSA) are largely overlooked in daily practice. A 60-year-old man who gave no history of any systemic illness presented to us 1 week following an uneventful cataract surgery with posterior chamber intraocular lens implantation in his right eye. The surgery was performed elsewhere by an eye-healthcare professional where the patient presented primarily with a history of progressively worsening diminution of vision in the same eye for 5 days and was diagnosed with a senile cataract. The postoperative visual gain was unsatisfactory; hence he sought another opinion. A diagnosis of non-arteritic AION (NAION) was established. Systemic evaluation revealed elevated diastolic blood pressure, dyslipidaemia and severe OSA. Prompt treatment with systemic steroids and simultaneous management of the accompanying systemic morbid conditions saved some useful vision in the affected eye. This also prevented involvement of the fellow unaffected eye. A comprehensive ocular examination with emphasis on systemic evaluation of the patient for coexisting illness is imperative before proceeding with any medical or surgical intervention. OSA is a definitive risk factor for the development of NAION, though it remains underdiagnosed and untreated. Cataract surgery has been shown to worsen underlying NAION. Systemic stabilization averts potentially blinding sequel in the unaffected eye of these patients.
INTRODUCTION
Decreased blood supply to the optic disc and anterior portion of the optic nerve by the posterior ciliary arteries causes anterior ischaemic optic neuropathy (AION). Non-arteritic AION (NAION) is the more common of the two subtypes, with an estimated annual incidence of 2.3/100 000, making it the most common acute optic neuropathy in the elderly.1,2 The typical clinical picture is rapid onset painless visual loss in an elderly patient with vasculopathic risk factors.1 The pathophysiology of NAION remains unclear. Hayreh categorized the risk factors as predisposing and precipitating factors.2 Apart from a disc at risk with a small size optic disc and small cup-to-disc ratio, systemic predisposing factors frequently associated with NAION include systemic arterial hypertension, diabetes mellitus, atherosclerosis, raised diastolic blood pressure, arteriosclerosis, vasospastic disorders, sleep apnoea and thromboembolic disorders.1–5 Smoking can affect the ability of the optic nerve head to autoregulate its blood flow, making it vulnerable to ischaemia.
THE CASE
A 60-year-old man who was a chronic smoker developed progressively worsening visual acuity (VA) in one eye. A diagnosis of NAION with multiple systemic comorbid conditions was established when he presented with an unsatisfactory visual gain following a cataract surgery with intraocular lens (IOL) implantation performed elsewhere in an attempt to visually rehabilitate him. He presented to us with unilateral sudden diminution of vision in his right eye (OD) for the past 12 days. The deterioration in his VA was progressive to the extent that he could only count fingers (FC) at a distance of 2 metres. At the onset of this acute event, he sought consultation elsewhere where he was diagnosed with senile cataract as the cause for this reduction in VA and he underwent phacoemulsification, with a foldable IOL implantation under topical anaesthesia, 5 days after the onset of symptoms. The surgery was uneventful. However, the postoperative visual gain was negligible. According to the patient, there was further deterioration in his VA OD after surgery compared to the preoperative status to the extent that he could FC at 1 metre when he presented a week later to a tertiary eye care centre. His medical records suggested that he was using bifocal high power glasses of +7.00 dioptre spheres (DS) for distance with a near addition of +2.50 DS in both eyes (OU) for the past 20 years. He possibly had ametropic amblyopia, a functional disorder where the VA is low in both eyes due to symmetrical and comparable high refractive error usually hypermetropia in either eye that goes uncorrected in the early years of childhood, and there is no other discernable organic cause for this low VA. Further, there was a history of poor vision OD since childhood in this patient.
At presentation, his best corrected VA (BCVA) with –0.75 diopter cylinder × 90º was FC at 1 metre OD. This change in refractive error was due to the IOL implant following cataract surgery. His BCVA with +7.00 DS was 6/18 in the left eye (OS). Projection of rays was inaccurate in all the quadrants OD but accurate OS. Ocular adnexa examination revealed xanthelasma on bilateral upper eyelids. A relative afferent pupillary defect was observed OD. Anterior segment examination was unremarkable OU except for the presence of a clear corneal incision at 12 o’clock of the recent cataract surgery OD. There was no evidence of uveitis OU. Fundoscopy revealed a swollen right optic disc with peripapillary splinter haemorrhages (Fig. 1a). The optic disc OS was crowded with a ‘disc at risk’ configuration (Fig. 1b). There was focal arteriolar attenuation and arteriovenous cross-over changes suggestive of hypertensive retinopathy (OU) (Fig. 1a and b). Intraocular pressure (IOP) measured using Goldmann applanation tonometer was 14 mmHg OU. The patient could not identify any plate of the Ishihara color vision charts from his right eye, unlike his left eye from which he could recognize 17/17 plates. The PelliRobson score was 0.00 OD suggesting complete loss of contrast sensitivity whereas contrast sensitivity OS was 1.80. Fundus fluorescein angiography revealed features of NAION OD (Figs 1c and d).
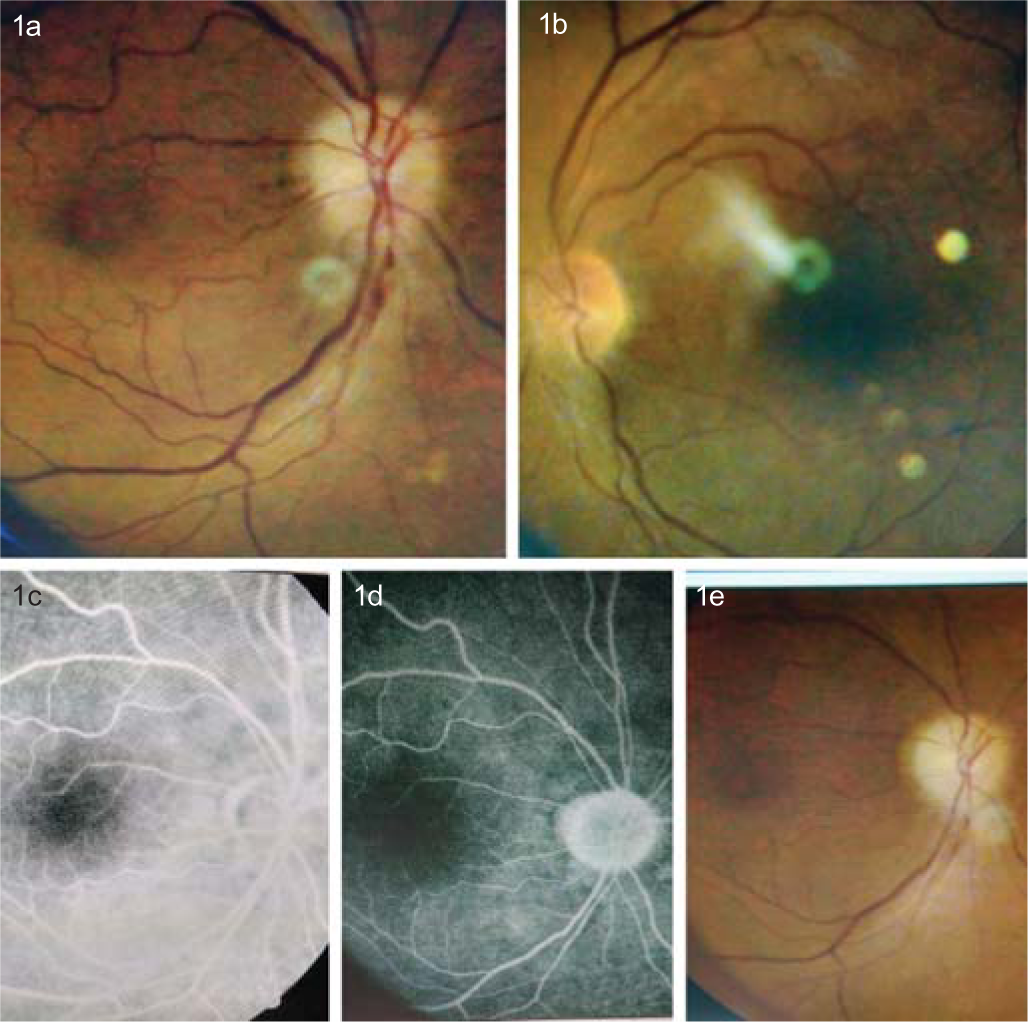
- Sequential fundus photographs and fundus fluorescein angiography images. Optic disc of the right eye showed swelling with peripapillary splinter haemorrhages (a). The left eye showed grade 2 hypertensive retinopathy with a small, crowded disc (b). Fundus fluorescein angiography showed normal filling of the peripapillary choroid with late staining at the optic nerve head (c and d). Right optic disc pallor seen at later stages (e)
The patient did not have any history of diabetes, hypertension, breathing disorder, cerebrovascular event or coronary artery disease and did not report weight loss, fever, headache or depression. At presentation, he had high blood pressure (150/90 mmHg) and tablet amlodipine (10 mg daily) was started. The blood glucose (105 mg/dl), erythrocyte sedimentation rate (ESR; 18 mm in the first hour), serum glycosylated haemoglobin (HbA1c 6.0%) and C-reactive protein were within normal limits. However, total serum cholesterol, low-density lipoprotein cholesterol, and triglyceride levels were 319 (normal 125–240), 189 (normal <150), and 195 (normal 50–150) mg/dl, respectively. Serum aspartate aminotransferase and alanine aminotransferase levels were normal. Anti-nuclear antibodies and anti-phospholipid antibodies were negative. Thyroid function tests, screening for syphilis, chest X-ray and contrast-enhanced computed tomography of the thorax were all normal. To investigate the cause for NAION, polysomnography was done and revealed severe obstructive sleep apnoea (OSA; apnoea–hypopnoea index 64.8/h) with adequate continuous positive airway pressure (CPAP) titration achieved at 12 cm H2O. Thus, a diagnosis of NAION with severe OSA, hyper-tension with dyslipidaemia was made.
The patient was advised lifestyle modifications including weight reduction, CPAP of 12 cm H2O and avoid supine posture while sleeping. He was started on oral prednisolone 60 mg/day. Rosuvastatin (40 mg once daily) and aspirin (75 mg once daily) were also added after consultation with a neurologist. VA (OD) progressively improved to 6/24 in 6 weeks with resolution of right optic disc swelling. However, there was development of disc pallor (Fig. 1e). Severe visual field (VF) loss with a mean deviation of –28.46 dB and large scotoma in the central 30º OD was observed on VF charting. Humphrey’s VF analysis revealed a peripheral rim defect with fixation losses OS. Oral steroids were gradually tapered over 8 weeks and then stopped. Further, the morphology of the visual field defects at the 2nd week was similar to that in the 1st week, with a lower mean deviation.
DISCUSSION
Swelling of even a few nerve fibres of the optic nerve can lead to compressive death of other fibres in the small crowded discs, which are appropriately labelled as ‘disk at risk’.3
OSA is characterized by repeated episodes of partial or complete upper airway obstruction leading to the cessation of breathing during sleep.6 The Wisconsin Sleep Cohort Study reported that ‘the apnoea–hypopnoea index (the average number of disordered breathing events per hour of sleep) is an independent predictor of daytime hypertension, with an odds ratio of 2.89 among patients with an apnoea–hypopnea index >15/h’.7 The exact mechanism by which OSA leads to NAION is only vaguely understood. Nocturnal apnoeic spells in individuals with OSA lead to a cycle of hypoxia and reoxygenation resulting in the release of free radicals which damage the vascular endothelium and thus affect blood flow autoregulation of the optic nerve head.8 This impaired autoregulation makes the optic nerve susceptible to ischaemic damage. It is also suggested that apnoeic spells lead to increased intracranial pressure (ICP) and thus damage the optic nerve directly.9 Various studies linking OSA to idiopathic intracranial hypertension (IIH) have found marked nocturnal increases in ICP related to apnoeic spells in OSA patients, while their daytime reading remains within normal limits.10–12 Investigators proposed that episodic nocturnal hypercapnia and hypoxia induce cerebral vasodilatation and this leads to increased cerebral blood flow and raised ICP.9 Thus, nasal CPAP during sleep is an indirect treatment for NAION in patients with OSA. It acts as an ‘pneumatic splint’ to keep the airways open.6 By reducing the nocturnal hypercapnia and hypoxia, CPAP can potentially reduce the incidence of NAION in such patients. Other management strategies of OSA include weight loss and upper airway/oropharyngeal surgery.6
Several landmark studies have contributed tangible evidence proving the association between OSA and NAION as well as the efficacy of treatment in preventing the development of OSA. Mojon et al. in 2002 did a cross-sectional case-controlled study using respiratory disturbance index on overnight polysomnography as an aid to diagnose and grade OSA in 17 patients and 17 controls.9 Twelve (71%) of 17 patients with NAION were diagnosed with OSA versus only 3 (18%) among controls. Of these 12 patients, 4 (24%) each had mild, moderate and severe OSA. Palombi et al. in 2006 studied the prevalence of OSA in 27 freshly diagnosed cases of NAION using the same index in an overnight sleep study.8 They compared the prevalence of OSA in these patients to that found in the general population in an earlier study. Twenty-four (89%) patients were diagnosed with OSA. Patients with NAION were observed to have a higher risk of OSA compared to the general population (risk ratio 4.9).
Li et al. in 2007 evaluated 73 patients of NAION and 73 controls who were assessed for symptoms of OSA using the sleep apnoea scale of the Sleep Disorders Questionnaire instead of overnight polysomnography.13 Patients with NAION were substantially more likely than controls to report features associated with OSA. OSA is associated with multiple conditions namely normal tension glaucoma (NTG), NAION apart from other disorders. Stein et al. in 2011 in a retrospective longitudinal cohort study compared the incidence of open-angle glaucoma, NTG, papilloedema, NAION and idiopathic intracranial hypertension (IIH) among persons with and without OSA as well as patients on treatment with CPAP versus those not on treatment.14 They found no significant relationship between sleep apnoea (either treated or untreated with CPAP) and the development of OAG or NTG. Patients with OSA not on treatment with CPAP had higher chances of developing NAION and IIH in contrast to controls without OSA. However, corresponding increased risk was not observed for OSA patients on treatment with CPAP.15 Non-conformance to CPAP in patients with NAION and severe OSA amplified the risk of involvement of the fellow eye (hazard ratio 5.54) in 67 of 89 patients with NAION who had accompanying OSA based on the findings of a prospective study done by Aptel et al.16 Sun et al. in a retrospective, longitudinal cohort-based study calculated the incidence of optic neuropathy in OSA patients aged over 20 years versus non-OSA controls for 8 years. There was 1.95-fold higher risk of developing optic neuropathy relative to controls in patients with OSA.17 The hazard ratios of the optic neuropathy risk were 1.82, 2.31 and 2.42 for OSA patients without treatment, treated with CPAP only and treated with surgery only, respectively, when compared with non-OSA controls. Yang et al. studied 919 patients with OSA over the age of 40 years, each matched with 10 controls without OSA.18 These subjects were followed retrospectively over 10 years to look for the development of NAION. The patients with OSA had an increased 10-year incidence of developing NAION when compared to controls.
Conversely, few studies have doubted the NAION–OSA relation. Peter et al. used a questionnaire on visual symptoms and fundoscopic examination to determine the prevalence of papilloedema in newly diagnosed patients with OSA. None of the 35 subjects developed papilloedema and they concluded that ‘systematic screening of papilloedema in these patients does not seem to be warranted and should be restricted to patients with visual complaints’.19 In their retrospective study of patients with NAION aged 40–75 years, Cestari et al. found several modifiable risk factors to be associated with the disease such as older age, male gender, Caucasians, hypertension, hypercoagulable states, end-organ involvement from diabetes, age-related macular degeneration and retinal vein occlusion.20 However, OSA was not identified as an independent risk factor of NAION.20
Our patient may require intervention for senile cataract in his fellow eye in due course. The association between cataract surgery and NAION is well documented in the literature.21–24 Hayreh recognized two different categories of NAION post-cataract surgery—the ‘immediate-type’ occurring hours after surgery and the ‘delayed-type’ occurring days to weeks after surgery.25 Increased IOP has been invariably implicated as a causative factor of the ‘immediate-type’. The pathogenesis of ‘delayed type’, in contrast, remains foggy—the most acceptable theory being proinflammatory factor-mediated vasculopathy in the postoperative period leading to compromised optic disc blood flow in susceptible individuals, thus triggering disc oedema.25 While various studies suggested that a history of NAION in the other eye may be a risk factor for this event, Lam et al. tried to quantify this through their retrospective study.22–25 They concluded that cataract surgery in the fellow eye of patients with prior history of contralateral NAION increased the risk of NAION in the fellow eye nearly 4 times. However, they did not comment upon the possible decrease in risk as time passes after cataract surgery.26
It is imperative to recognize this possible complication, however infrequent, and educate patients before surgery. It is arguable that we avoid doing cataract surgery in the fellow eye until low vision starts affecting the quality of life of the patient and even then, phacoemulsification by an experienced surgeon needs to be done to avoid unnecessary postoperative inflammation. In advanced cataract, where the fundus may not be adequately seen, pupil reactions can give a clue about the status of the optic nerve. In suspicious cases such as those with sluggish pupillary reactions and/or degree of vision loss not corresponding to the cataract grade, a visual-evoked potential may be advised. A tight control of postoperative IOP needs to be maintained as well.
Symptoms of OSA may go unnoticed by the patient and this condition must be addressed and investigated in patients presenting to a neurologist/neuro-ophthalmologist with clinical features of NAION. A decision for cataract surgery or any intraocular procedure must be taken with caution. Prompt holistic management saves useful vision in these patients.
Conflicts of interest
None declared
References
- Nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol. 2002;13:357-61.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23:157-63.
- [CrossRef] [PubMed] [Google Scholar]
- Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76-96.
- [CrossRef] [PubMed] [Google Scholar]
- Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603-24.
- [CrossRef] [PubMed] [Google Scholar]
- Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549-54.
- [CrossRef] [PubMed] [Google Scholar]
- Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429-37.
- [CrossRef] [PubMed] [Google Scholar]
- Nonarteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. 2006;90:879-82.
- [CrossRef] [PubMed] [Google Scholar]
- Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120:601-5.
- [CrossRef] [PubMed] [Google Scholar]
- Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogr Clin Neurophysiol. 1985;60:214-19.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial pressure and obstructive sleep apnea. Chest. 1989;95:279-83.
- [CrossRef] [PubMed] [Google Scholar]
- Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118:1626-30.
- [CrossRef] [PubMed] [Google Scholar]
- Nonarteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the Sleep Apnea scale of the Sleep Disorders Questionnaire (SASDQ) Br J Ophthalmol. 2007;91:1524-7.
- [CrossRef] [PubMed] [Google Scholar]
- The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152:989-98.
- [CrossRef] [PubMed] [Google Scholar]
- Nonarteritic anterior ischemic optic neuropathy in patients with sleep apnea while being treated with continuous positive airway pressure. Am J Ophthalmol. 2005;139:518-21.
- [CrossRef] [PubMed] [Google Scholar]
- Association of nonarteritic ischemic optic neuropathy with obstructive sleep apnea syndrome: consequences for obstructive sleep apnea screening and treatment. JAMA Ophthalmol. 2015;133:797-804.
- [CrossRef] [PubMed] [Google Scholar]
- Association between obstructive sleep apnea and optic neuropathy: A Taiwanese population-based cohort study. Eye (Lond). 2018;32:1353-8.
- [CrossRef] [PubMed] [Google Scholar]
- Obstructive sleep apnoea and increased risk of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2019;103:1123-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of papilloedema in patients with sleep apnoea syndrome: A prospective study. J Sleep Res. 2007;16:313-18.
- [CrossRef] [PubMed] [Google Scholar]
- Demographic, systemic, and ocular factors associated with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2016;123:2446-55.
- [CrossRef] [PubMed] [Google Scholar]
- Neuro-ophthalmologic complications of cataract surgery. Semin Ophthalmol. 2002;17:149-52.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of nonarteritic anterior ischemic optic neuropathy associated with cataract extraction. Ophthalmology. 2001;108:1275-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nonarteritic anterior ischemic optic neuropathy and surgery of the anterior segment: Temporal relationship analysis. Am J Ophthalmol. 2003;136:1171-2.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of nonarteritic anterior ischemic optic neuropathy after cataract surgery. Am J Ophthalmol. 2019;207:343-50.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior ischemic optic neuropathy. IV. Occurrence after cataract extraction. Arch Ophthalmol. 1980;98:1410-16.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of non-arteritic anterior ischaemic optic neuropathy (NAION) after cataract extraction in the fellow eye of patients with prior unilateral NAION. Br J Ophthalmol. 2007;91:585-7.
- [CrossRef] [PubMed] [Google Scholar]




