Translate this page into:
Outcomes of patients with thrombotic thrombocytopenic purpura treated in an intensive care unit
Correspondence to KIRAN KUMAR GUDIVADA; gkiran17medico@gmail.com
[To cite: Narayan SK, Gudivada KK, Sivakoti S, Krishna B. Outcomes of patients with thrombotic thrombocytopenic purpura treated in an intensive care unit. Natl Med J India 2023;36:295–300. DOI: 10.25259/NMJI_205_21]
Abstract
Background
Thrombotic thrombocytopenic purpura (TTP) is a multisystem disorder characterized by widespread microthrombosis that can predispose to multiple organ failure. The literature is sparse on the outcomes of critically ill patients with TTP managed in intensive care units (ICUs). We aimed to determine the mortality of ICU patients admitted with TTP and evaluate the predictors of survival. We also compared the incidence of nosocomial infection among those who did or did not receive plasma exchange (PE).
Methods
We conducted a retrospective study in a tertiary ICU. Two authors screened patients for eligibility from the hospital information system based on peripheral smear reports. Adult critically ill TTP patients managed in ICU were included. Patients with a diagnosis of haemolytic uraemic syndrome, autoimmune causes of haemolysis and pregnancy-related conditions, etc. were excluded. Two authors extracted data from medical charts. No imputation of missing variables was done. Non-parametric statistics were used to report data. Statistical analyses were performed using Stata version 16.
Results
Of the 535 records that were screened, 33 patients were deemed eligible. Mortality among TTP patients was 14 (42%). The women to men ratio was 7:3. At admission, greater degree of anaemia, thrombocytopenia, and higher lactate dehydrogenase levels were observed in non-survivors compared to survivors (5.4 g/dl [4.8–7.1] v. 7.6 g/dl [6.1–8.9], p=0.05; 17x103 μl v. 21x103 μl, p=0.63; and 2987 (1904–3614) U/L v. 2126 U/L (1941–3319), p=0.71; respectively]. Nineteen (57%) patients had acute kidney injury (AKI), of which 11 survived: 6 recovered completely from renal failure and 5 progressed to end-stage renal disease. Nosocomial infection rates were not different among those receiving and not receiving PE therapy (7 [33%] v. 3 [25%], respectively).
Conclusion
TTP is more common in women and has a high mortality. Older age, low haemoglobin and higher platelet transfusions are predictors of poor survival. Nosocomial infection rates were similar irrespective of receiving PE therapy.
INTRODUCTION
Thrombotic microangiopathy (TMA) is a pathological diagnosis but often inferred from the presence of microangiopathic haemolytic anaemia (MAHA) and thrombocytopenia. Thrombotic thrombocytopenic purpura (TTP) is one of the important causes of primary TMA syndromes and a haematological emergency with mortality nearing 90% without specific treatment.1 TTP has additional clinical presentations in the form of fever, neurological and renal manifestations. However, presence of acute kidney injury (AKI) as a predominant feature was considered to favour the diagnosis of haemolytic uraemic syndrome (HUS).2
Patients with TTP may require intensive unit care (ICU) admission for organ failures or need of plasma exchange (PE) therapy. They are also at high risk to acquire nosocomial infections, especially, central line-associated bloodstream infection (CLABSI) due to the need of central venous access to perform PE.3,4 They frequently receive steroids or other immunosuppressants as a part of their disease treatment. All these factors predispose them to nosocomial infections and consequently progression to sepsis and multi-organ failure. In the ICU settings, differentiation between TTP and other causes of TMA such as HUS, atypical HUS, HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome and preeclampsia and sepsis is challenging due to considerable overlap of laboratory parameters and clinical features.5 In such cases, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13) protease activity testing is invaluable but non-availability and high cost of this test makes prediction models such as PLASMIC score more practical to predict the deficient activity of ADAMTS13 enzyme.6,7
The literature on critically ill TTP patients admitted to ICU is sparse. Studies in the Indian population are limited to only a few reported cases.8 Further, there is scanty literature describing the burden of nosocomial infections with the use of PE therapy and corticosteroids in the ICU in patients with TTP.9 We aimed to determine the cause of death that is attributable or non-attributable to TTP and evaluate the predictors of mortality in patients with TTP admitted to ICU. We also proposed to compare the incidence of nosocomial infection among those receiving PE with those not receiving it.
METHODS
We did a retrospective study in a 30-bedded medical-surgical ICU of an Indian tertiary care hospital after obtaining permission from the institute ethical committee (IEC Study Reference No. 119/2020).
Patients
Consecutive patients admitted to the ICU between December 2012 and June 2020 with a diagnosis of probable TTP at admission or during hospitalization were included. ‘Probable TTP’ was defined as the presence of thrombocytopenia and MAHA along with evidence of visceral organ involvement (cardiac, renal, neurological, gastrointestinal, etc.) and no alternative aetiology established to explain the gamut of clinical presentation. The diagnosis of probable TTP was made by the treating physician in consultation with a haematologist. (For the rest of the paper, we refer to ‘probable TTP’ as TTP.)
Patients were screened for eligibility with the help of the hospital information system (HIS). Inclusion criteria were patients’ age more than 18 years, those suspected to have TTP and managed in ICU. The following search terms ‘schistocytes’, ‘fragmented cells’, ‘micro-angiopathic haemolytic anaemia’ were used to screen peripheral smear reports in our HIS. Two authors (GK and SK) screened the records and excluded patients whose diagnosis was HUS, atypical HUS, autoimmune causes of haemolysis, snakebite, accelerated hypertension, malignancies, septic shock, disseminated intravascular coagulation, vasculitis, and pregnancy-related causes such as pre-eclampsia, HELLP syndrome and acute fatty liver of pregnancy.
Study data, definitions and outcome measures
Two authors (GK and SK) extracted the following data from the medical records: demographic parameters, medical history, presenting symptoms, admission APACHE (acute physiology and chronic health evaluation) II, daily SOFA (sequential organ failure assessment) score, interventions performed such as PE, use of adjuvant steroids and plasma infusion. The presenting symptoms were classified as cardiac (myocardial infarction, non-ST elevation myocardial infarction [NSTEMI], left ventricular dysfunction, arrythmias), renal (proteinuria, haematuria, oliguria, anuria, fluid overload), neurological (stroke, seizures, coma, focal deficits, headache, visual disturbances), gastrointestinal (vomiting, diarrhoea, abdominal pain), and thrombotic symptoms (peripheral vascular thrombosis). Since the ADAMTS 13 activity was not measured, due to lack of availability of the test, the risk of ADAMTS 13 deficiency was predicted using PLASMIC score, which was calculated from the available data.6 This clinical predictive tool contains seven variables. A score of ‘one’ was credited for each variable for platelet count <30 000/cmm, haemolysis (reticulocyte count >2.5% or haptoglobin undetectable or indirect bilirubin >2 mg/dl), active cancer (treated for cancer within the past year), history of solid organ or stem cell transplant, mean corpuscular volume <90 fl, INR <1.5 and creatinine <2 mg/dl. A cumulative score of 6–7 was considered to predict severe ADAMTS 13 deficiency.6
Clinical outcomes such as mortality in ICU, length of ICU and hospital stay, and days on invasive mechanical ventilation and nosocomial infection rates were recorded. Nosocomial infection or healthcare-associated infection was defined as an infectious event diagnosed after admission of the patient to hospital and with no evidence that the pathogen was already in the incubation period at admission. Central line-associated bloodstream infection (CLABSI) was defined as a laboratory confirmed infection where a central venous access was in place for >2 calendar days before a positive culture and was also in place the day of or day before culture, catheter-associated urinary tract infection (CAUTI) was defined as a urinary tract infection where an indwelling urinary catheter was in place for more than 2 consecutive days in an inpatient location on the date of event and infection-related ventilator-associated complication (IVAC) was defined as per the Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) surveillance definitions.10 AKI was defined based on KDIGO (Kidney Disease Improving Global Outcomes) 2012 clinical practice guideline as an increase in serum creatinine by >0.3 mg/dl within 48 hours; or an increase in serum creatinine to >1.5 times baseline, which is known or presumed to have occurred within the previous 7 days; or urine volume <0.5 ml/kg/hour for 6 hours.11
Data end-points and statistical methods
Patients were categorized into two groups based on mortality (survivors and non-survivors) and also based on treatment strategy (PE recipients and PE non-recipients) and predictor variables were evaluated in a bivariate model. Continuous variables were described as mean with standard error (SE) or median with interquartile range (IQR) and categorical variables as numbers with percentage. Due to the small sample population, non-parametric tests such as Fisher exact test to compare absolute numbers and percentages and Wilcoxon rank sum test to compare parameters expressed in median and IQR were preferred. Imputation of missing variables was not done. A p value of <0.05 was considered significant. Statistical analyses were performed using Stata version 16 (StataCorp, College Station, Texas 77845 USA).
RESULTS
Among 535 patients that were identified (age >18 years and haemolysis in peripheral smear) during the study period, 439 patients were excluded (direct Coombs-positive or not admitted to ICU). The remaining 96 patient-charts were thoroughly screened and 33 patients whose diagnosis was determined as probable TTP were included for the final quantitative analyses (Fig. 1). All the included patients were diagnosed to have TTP during their hospital admission. Individual patient details are given in supplementary Table I (available at www.nmji.in).
Mortality among TTP patients was 14 (42%). Preponderance of women was seen with women to men ratio of 7:3. Also, deaths among women were higher 12 (52%).
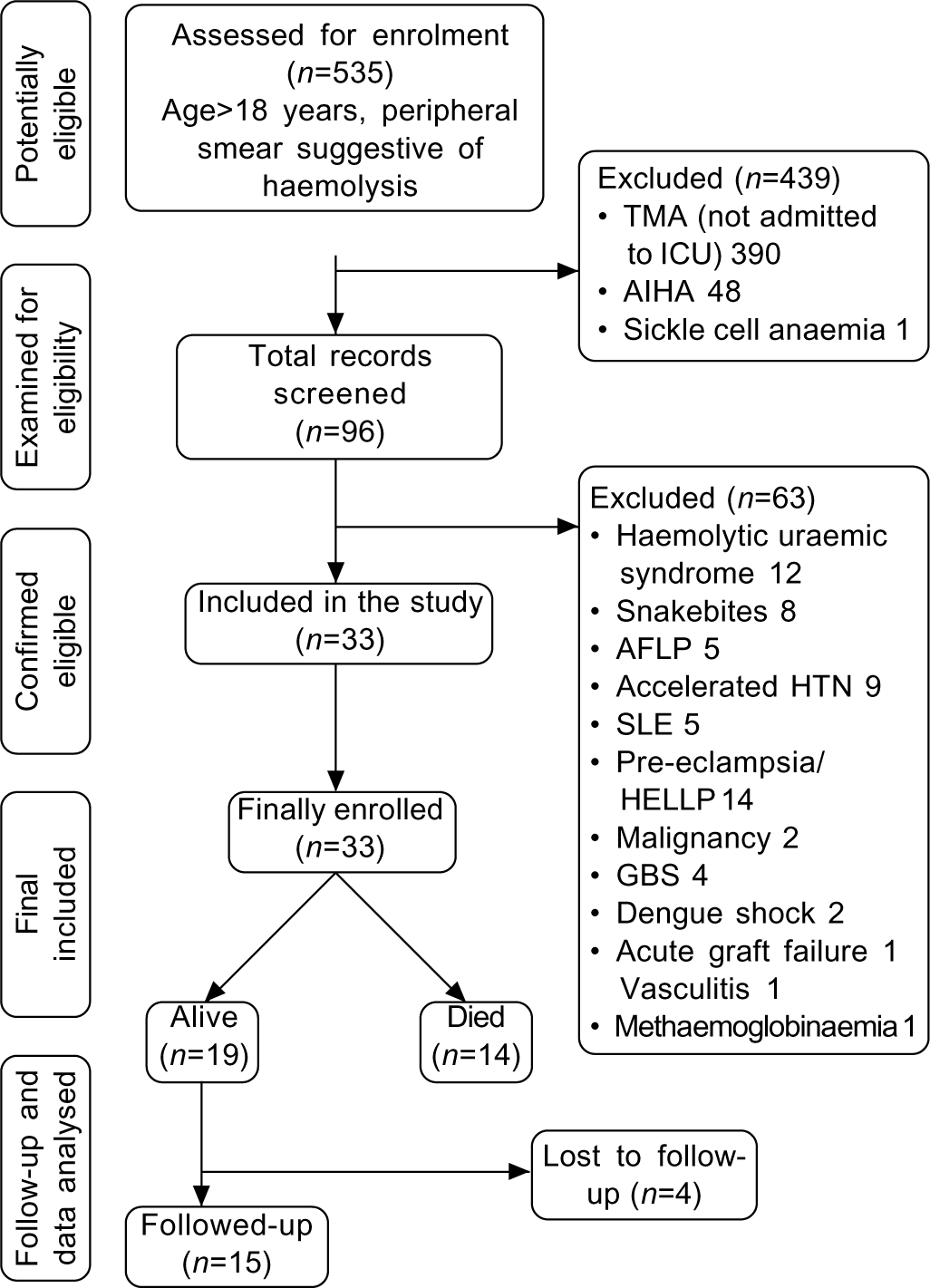
- Study flowchart TMA thrombotic microangiopathy HTN hypertension AIHA autoimmune haemolytic anaemia SLE systemic lupus erythematosus ICU intensive care unit HELLP heamolysis, elevated liver enzymes, low platelet count AFLP acute fatty liver of pregnancy GBA Guillain–Barre syndrome
Patients who died were significantly older than the survivors of TTP (42 [4.2] v. 30.6 [1.9] years, p=0.02). There were no statistically significant differences among survivors and non-survivors with respect to gender, body mass index, APACHE II score, day 1 SOFA score. Seven of 33 patients were detected to have hypothyroidism as a comorbid condition, of which 6 patients died. The PLASMIC score was also not statistically different among the survivors and non-survivors (5.5 [0.26] v. 5.7 [0.28], p=0.93; Table I).
| Characteristic | Alive (n=19) | Died (n=14) | p value* |
|---|---|---|---|
| Mean (SE) age (years) | 30.58 (1.9) | 42 (4.2) | 0.01 |
| Gender (women), n(%) | 11 (58) | 12 (86) | 0.08 |
| Mean (SE) body mass index | 25.7 (4.1) | 21.3 (0.4) | 0.37 |
| Comorbid conditions, n (%) | |||
| Diabetes mellitus | 1 (5) | 3 (21) | 0.28 |
| Hypertension | 4 (21) | 3 (21) | 0.97 |
| Hypothyroidism | 1 (5) | 6 (42) | 0.02 |
| Mean (SE) APACHE II | 15.7 (1.8) | 17.7 (2.3) | 0.5 |
| Mean (SE) day 1 ICU SOFA | 7.2 (0.6) | 1 1 (3.2) | 0.2 |
| Mean (SE) PLASMIC score | 5.5 (±0.3) | 5.7 (±0.3) | 0.73 |
| Platelet count at admission (per cmm), median (IQR) | 2 1 5 0 0 (8000–59 000) | 1 7 0 0 0 (10 500–71 500) | 0.64 |
| Lactate dehydrogenase at admission U/L, median (IQR) | 2126 (1941–3319) | 2987 (1904–3614) | 0.71 |
| Haemoglobin at admission in g/dl, median (IQR) | 7.6 (6.1–8.9) | 5.4 (4.8–7.1) | 0.05 |
| Total bilirubin, median (IQR) | 2.4 (1.09–5.27) | 2.25 (1.62–3.8) | 0.96 |
| Reticulocyte count, median (IQR) | 12.8 (6.6–16.5) | 8.1 (4.5–10.1) | 0.42 |
| Creatinine at admission, median (IQR) | 2.13 (1.2–6.6) | 1.52 (0.8–3.7) | 0.22 |
| Acute kidney injury during hospitalization, n(%) | 11 (58) | 8 (57) | 0.62 |
| Days from symptom onset to ICU admission, median (IQR) | 7 (3–10) | 8.5 (7–11) | 0.15 |
| Days from symptom onset to hospital admission, median (IQR) | 7 (3–10) | 7.5 (3–10) | 0.20 |
| Fever at presentation, n(%) | 11 (57.8) | 7 (50) | 0.73 |
| Neurological presentation, n(%) | 13 (68.4) | 10 (71) | 1.0 |
| Cardiac presentation, n(%) | 1 (0.5) | 3 (21) | 0.28 |
| Gastrointestinal presentation, n(%) | 12 (63) | 6 (43) | 0.34 |
| Haemorrhagic presentation, n(%) | 3 (16) | 6 (43) | 0.12 |
| Renal presentation, n(%) | 16 (84) | 10 (71) | 0.4 |
| Thrombotic manifestation, n(%) | 1 (5) | 2 (14) | 0.5 |
| PE (plasma exchange), n(%) | 14 (74) | 7 (50) | 0.27 |
| Day of PE of mean (SE) | 1.5 (0.3) | 1.1 (0.3) | 0.38 |
| Cumulative volumes (L) of PE among patients who had PE, median (IQR) | 8.7 (5–13.5) | 6 (2–6.5) | 0.08 |
| Only fresh frozen plasma transfusion without PE, n(%) | 2 (10) | 3 (21) | 0.62 |
| Received steroids, n(%) | 11 (58) | 10 (71) | 0.33 |
| Total dose of steroids equivalent to methyl prednisolone (mg), mean (SE) | 457 (135) | 31 1 (122) | 0.43 |
| Number of packed blood cell transfusion, mean (SE) | 4.7 (0.5) | 2.4 (0.6) | 0.01 |
| Number of platelet transfusion, mean (SE) | 1.3 (0.5) | 6.6 (2.6) | 0.02 |
| Need for renal replacement therapy, n(%) | 9 (47) | 7 (50) | 0.58 |
| Total hospital acquired infections, n (%) | 4 (21) | 8 (57) | 0.06 |
| CAUTI | 0 | 2 | – |
| CLABSI | 3 | 1 | – |
| IVAC | 1 | 3 | – |
| HAP | 0 | 2 | – |
| Complications related to TTP, n(%)† | 10 (53) | 12 (86) | 0.06 |
Laboratory variables
There was a trend towards greater severity of anaemia, thrombocytopenia, and higher lactate dehydrogenase levels at admission in patients who died compared to those alive (5.4 g/dl [4.8–7.1] v. 7.6 g/dl [6.1–8.9]; p=0.05, 17 000/cmm v. 21 000/cmm, p=0.63 and 2987 (1904–3614) U/L v. 2126 U/L (1941–3319); p=0.71, respectively; Table I). Other baseline laboratory parameters such as reticulocyte count, serum creatinine and serum bilirubin levels were not significantly different among the two groups.
Intervention measures
Time to symptom onset to ICU admission was similar in the two groups (7 [3–10] days v. 8.5 [7–11] days; p=0.22). A higher number of survivors than non-survivors received PE, although this was not statistically significant (14 [74%] v. 7 [50%]; p=0.27). Cumulative median plasma volume exchange was 8.7 (5–13.5) L in survivors v. 6 (2–6.5) L in non-survivors (p=0.08). Trend of mean SOFA scores after day 4 was higher in the non-PE group than the PE group (Fig. 2). The mean dose of steroids was 457 (135) mg v. 311 (122) mg of methylprednisolone equivalent in those who survived and died, respectively. Platelet transfusion was significantly lower in survivors than non-survivors (1.3 [0.5] v. 6.6 [2.6] units, p=0.02), while packed red blood cell transfusion was higher in survivors than non-survivors 4.7 [0.5] v. 2.4 [0.6] units, respectively (Table I).
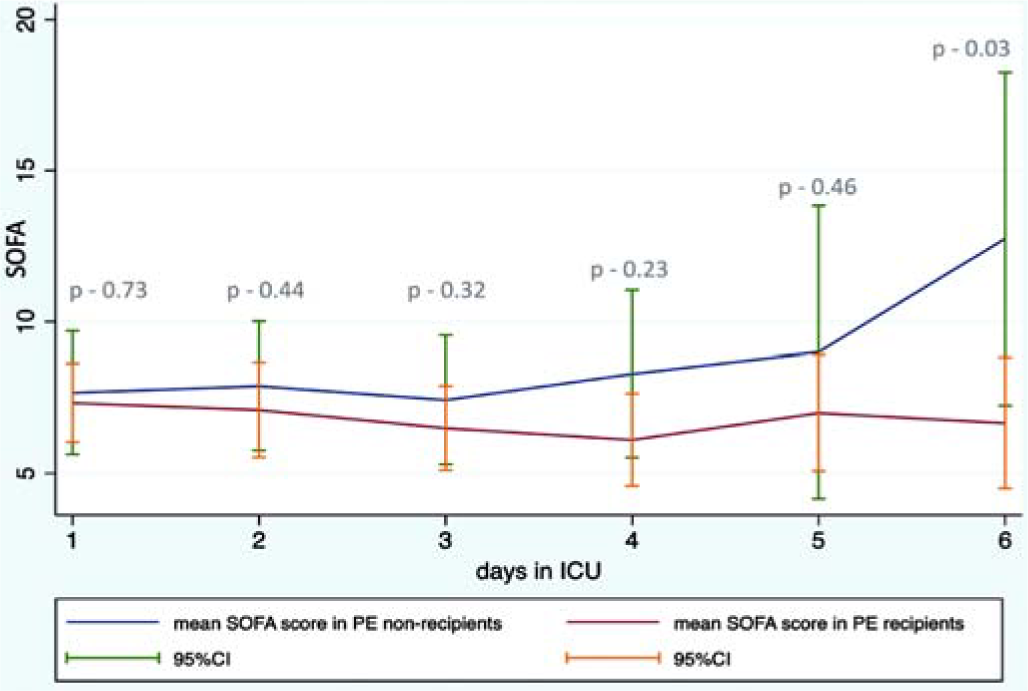
- Mean SOFA trend from day 1 to day 6 among those who received plasma exchange (PE) and those who did not SOFA sequential organ failure assessment
Clinical presentations and complications
Overall, 17 (51%) patients had fever at admission to the hospital. Renal 26 (78%), neurological 23 (69%) and gastrointestinal 18 (54%) were the three most common manifestations at admission followed by cardiac 4 (12%) and peripheral vascular thrombosis 3 (9%). Microscopic haematuria and proteinuria were the most common renal manifestations while seizure was the most frequent neurological presentation. Intra-hospital complications are given in Table II. Cause of death was directly attributed to TTP in 7 patients (Supplementary Table I).
| Complication | n |
|---|---|
| Refractory status epilepticus | 4 |
| Acute severe pancreatitis | 4 |
| Hyperkalaemia cardiac arrest (due to haemolysis) | 4 |
| Acute cerebrovascular accident | 3 |
| Cerebral oedema | 3 |
| Mesenteric ischaemia | 2 |
| Anaphylactic reaction to fresh frozen plasma | 1 |
Follow-up of AKI and non-AKI survivors
Acute kidney injury occurred in 57% (n=19) of our cohort, of which 11 survived. Six patients completely recovered from renal failure while 5 others progressed to end-stage renal disease (ESRD) requiring long-term dialysis (Supplementary Table I). None of them had recurrence of TTP at 1 year follow-up. On follow-up at 1 year, of the 8 non-AKI survivors, 4 patients were disease-free and 4 others were lost to follow-up.
Risk of nosocomial infections
The incidence of CAUTI, CLABSI, IVAC and HAP were not different among PE and non-PE recipients (Table III). Overall, the incidence of infections was 7 (33.3%) and 3 (25%) in the PE and non-PE groups, respectively. ICU mortality was 7 (33%) in the PE and 7 (58%) in the non-PE groups.
| Infection | PE done (n=21) | No PE (n=12) | p value* |
|---|---|---|---|
| HAI | 9 (42) | 3 (25) | 0.45 |
| CAUTI | 1 | 1 | |
| CLABSI | 2 | 2 | |
| IVAC | 3 | 0 | |
| HAP | 3 | 0 | |
| ICU mortality | 7 (33) | 7 (58) | 0.27 |
DISCUSSION
TTP is a life-threatening haematological emergency with a high mortality unless treated early. Early initiation of PE therapy is an important strategy to improve survival.12 Many patients require ICU admission due to TTP-related complications and organ dysfunction.1 In our retrospective study of 33 critically ill patients with TTP, the mortality was 42%, which was higher than that reported in previous studies.12 The higher mortality in our study can be attributed to our study population being critically ill and admitted to ICU with complications of the disease. Of the 14 deaths, 7 (50%) were directly attributed to TTP-related complications such as pulmonary thromboembolism, hyperkalaemia cardiac arrest, status epilepticus and refractory intracranial hypertension. The cause of death for most other patients was related to hospital-acquired infections leading to sepsis and multi-organ failure. Nosocomial infections, which pose a greater challenge during hospitalization, were not statistically different between PE and non-PE recipients.
The severity of illness scores (APACHE II and SOFA) was not significant between survivors and non-survivors implying that severity of illness at admission did not affect outcomes. We observed a gender predominance and a higher mortality among women. Similar findings were reported in the Southeast England registry for TTP.13
Accurate diagnosis of TTP was not possible in our study due to non-availability of ADAMTS 13 activity assays. In many developing countries including India this assay is not widely available and limited by high cost and long turnaround time. Multiple studies have shown that severe ADAMTS 13 deficiencies (activity <10% of normal) was not found in all patients who were clinically diagnosed with acute TTP,14 thus a high degree of clinical suspicion adds value to the diagnosis. However, in cases of a clinical dilemma, ADAMTS 13 assay is invaluable in differentiating TTP from other causes of TMA. In a UK registry-based study, Hassan et al. showed nearly 20% overlap of laboratory parameters between TTP and other causes of TMA.15 We may have missed few cases of TTP, especially those who had an overlap with autoimmune aetiologies and TTP during pregnancy whose diagnosis is possible only with demonstration of low ADAMTS 13 activity.16 PLASMIC score was comparable among survivors and non-survivors in our study and was not found useful in predicting mortality. The mean PLASMIC score of our study was 5.6 and it has been shown that scores <6 had inadequate sensitivity to rule out TTP.7
Renal, neurological and gastrointestinal manifestations were the three most common presentations of our patients. Renal manifestations include proteinuria in 21 (63.6%) and microscopic haematuria in 19 (57.6%) patients which signify the presence of focal or segmental glomerular microthrombi.17 The incidence of AKI in our study was 57.6%, including 73.7% with stage 3 AKI. This is comparable with the incidence of AKI seen in a study by Zafrani et al.18 However, stage 3 AKI requiring dialysis was much higher in our study. On follow-up, of 11 AKI survivors, 5 progressed to ESRD and 3 patients had delayed renal recovery between 6 to 12 months. The other 3 recovered from AKI during the ICU stay. Therefore, we recorded a 45% risk of progression of AKI to ESRD. These results show that the incidence of AKI and progression to chronic kidney disease is much higher than previously believed. Therefore, a presumption that severe renal failure excludes the diagnosis of TTP to favour HUS can mislead physicians and, consequently, delay the initiation of PE therapy. The most frequent neurological presentation was seizures (36.4%), among these one-third had refractory status epilepticus and few others had acute stroke. Hyperkalaemic cardiac arrest was identified in 4 patients; among them 2 patients were found to have ongoing haemolysis despite PE therapy.
A striking finding in our TTP cohort was the high prevalence of hypothyroidism (21%). There are a few case reports showing an association between TTP and hypothyroidism.19 The possible explanation given was thrombotic microangiopathy to the vascular supply of thyroid gland leading to hypothyroidism. However, our patients had hypothyroidism before the hospital admission. Further work-up is required to ascertain this association and delineate the pathophysiology.
PE and adjuvant steroid therapy are the mainstay of treatment of TTP. Cumulative median plasma volume exchanges in our cohort were lower than recommendations provided in the literature.20 Due to resource limitations, on most occasions, PE therapy in our patients were terminated after assessing for clinical improvement (such as improvement in Glasgow Coma Scale and other organ functions). Among patients who did not receive PE following reasons were cited in the medical charts: refractory septic shock, immediate demise upon admission, acute coronary syndrome, pulmonary thromboembolism and lack of consent. We noticed higher platelet transfusions in non-survivors. Similar observations were noted by Goel et al. in a large database study where they found higher odds of arterial thrombosis, myocardial infraction and deaths in TTP patients with platelet transfusions.21
The burden of nosocomial infections is higher in ICUs and this could be a major hindrance to the initiation and continuation of PE therapy. CLABSI is a concern in these patients due to frequent handling of vascular access during the procedure. Overall, the incidence of CLABSI in our cohort was 12%, which was comparable with the other studies and the incidence was similar regardless of PE therapy.22,23 Other nosocomial infection rates such as CAUTI, IVAC and HAP were not significantly different in those who received PE therapy. However, interpretation of these data were limited by a small sample population and fewer events.
Of the 19 TTP survivors, we could follow-up 15 patients (79%) and all of them were disease-free for 1 year.
Clinical implications of our study and future research
Our study provides insights into the ICU outcomes of TTP patients that have been sparsely reported. The incidence of AKI is higher than previously believed; hence clinicians should be cautioned while differentiating TTP from HUS based solely on the absence of AKI. Prospective studies with long-term follow-up are required for further prognostication of renal function. CLABSI with PE therapy was not higher than the expected incidence in ICU. A spurious association between TTP and hypothyroidism was noted, but this finding needs to be evaluated in prospective studies before being disregarded.
Limitations
There are important limitations to our study. Due to the small sample population, most analyses lack statistical power to ascertain associations. The retrospective study design and inability to include patients before the inception of our HIS was also a limitation. Being a single-centre study, generalizability of the results is questionable to a certain extent. However, we have followed standard protocols in managing TTP patients such as early initiation of PE and adjuvant steroids, though patients received lower volumes of plasma. ADAMTS 13 activity was not measured and the diagnosis of TTP was solely based on the discretion of treating physician in consultation with a haematologist. Finally, follow-up of 4 patients was lacking, thus the relapse of TTP in these patients could not be assessed.
To summarize, our study results showed higher mortality among TTP patients admitted to the ICU (42%). Of all the deaths, half of them were attributed to TTP-related complications, while the other half were attributed to sepsis from hospital-acquired infections. Older age, lower haemoglobin levels at admission and higher platelet transfusions were predictors of poor survival. PE therapy did not significantly increase the risk of infections. AKI occurred in 58% of patients with TTP and among AKI survivors 45% progressed to ESRD.
Conclusion
The results of our study showed that TTP is more common among women and they also had a higher mortality. Nosocomial infections were not significantly greater in PE recipients when compared to non-recipients. The incidence of AKI in patients with TTP was much higher than expected. Platelet administration adversely affected the survival of patients with TTP.
Conflicts of interest
None declared
References
- Expert statement on the ICU management of patients with thrombotic thrombocytopenic purpura. Intensive Care Med. 2019;45:1518-39.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma exchange in clinical practice. Available at www.intechopen.com/books/plasma-medicine-concepts-and-clinical-applications/plasma-exchange-in-clinical-practice (accessed on 15 Feb 2021)
- [Google Scholar]
- Infective complications of plasma exchange: A prospective study. Arthritis Rheum. 1987;30:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombocytopenia in the ICU: Disseminated intravascular coagulation and thrombotic microangiopathies-what intensivists need to know. Crit Care. 2018;22:158.
- [CrossRef] [PubMed] [Google Scholar]
- Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: A cohort study. Lancet Haematol. 2017;4:e157-e164.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of the PLASMIC score in patients with suspected thrombotic thrombocytopenic purpura: A systematic review and meta-analysis. Transfusion (Paris). 2020;60:2047-57.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic thrombocytopenic purpura: A case series from a tertiary care centre in northern India. Blood. 2018;132(Suppl 1):5006.
- [CrossRef] [Google Scholar]
- Thrombotic thrombocytopenic purpura: From diagnosis to therapy. Curr Opin Crit Care. 2015;21:593-601.
- [CrossRef] [PubMed] [Google Scholar]
- CDC/NHSN Surveillance Definition of Healthcare-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. 2013. Available at www.socinorte.com/wp-content/uploads/2013/03/Criterios-de-IN-2013.pdf (accessed on 15 Feb 2021)
- [Google Scholar]
- CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-32. Erratum in: Am J Infect Control 2008;36:655
- [CrossRef] [PubMed] [Google Scholar]
- KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012(Suppl 2):1-138. Available at https://experts.umn.edu/en/publications/kidney-disease-improving-global-outcomes-kdigo-acute-kidney-injur (accessed on 15 Feb 2021)
- [Google Scholar]
- Regional UK TTP Registry: Correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142:819-26.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of ADAMTS13 in differentiating TTP from other acute thrombotic microangiopathies: results from the UK TTP Registry. Br J Haematol. 2015;171:830-5.
- [CrossRef] [PubMed] [Google Scholar]
- Advantages and limits of ADAMTS13 testing in thrombotic thrombocytopenic purpura. Blood Transfus. 2008;6:127-35.
- [Google Scholar]
- Acute renal failure is prevalent in patients with thrombotic thrombocytopenic purpura associated with low plasma ADAMTS13 activity. J Thromb Haemost. 2015;13:380-9.
- [CrossRef] [Google Scholar]
- Thrombotic thrombocytopenic purpura in hypothyroidism: An accidental association? Haematologica. 1982;67:625-9.
- [Google Scholar]
- Membrane-based therapeutic plasma exchange in intensive care. Blood Purif. 2021;50:290-97.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood. 2015;125:1470-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of quality indicators in an Indian intensive care unit using “CHITRA” database. Indian J Crit Care Med. 2017;21:841-6.
- [CrossRef] [PubMed] [Google Scholar]
- Health-care-associated infections: Risk factors and epidemiology from an intensive care unit in Northern India. Indian J Anaesth. 2014;58:30-5.
- [CrossRef] [PubMed] [Google Scholar]




