Translate this page into:
Prevalence and antimicrobial resistance pattern of Burkholderia cepacia at a tertiary care teaching hospital
Correspondence to NAIMIKABEN PATEL; drnaimikapatel@gmail.com
[To cite: Patel N, Dabhi C, Patel R, Singh S. Prevalence and antimicrobial resistance pattern of Burkholderia cepacia at a tertiary care teaching hospital. Natl Med J India 2025;38:9–11. DOI: 10.25259/NMJI_498_ 2022]
Abstract
Background
Burkholderia cepacia (B. cepacia) is the fourth most common pathogenic non-fermenting gram-negative bacilli isolated from clinical samples in hospitalized patients. It is an emerging opportunistic pathogen causing a wide range of infections in immunocompromised and hospitalized patients.
Methods
We did a retrospective observational study at Shree Krishna Hospital, Karamsad after approval by the Institutional Ethics Committee to determine the prevalence and antimicrobial resistance pattern of B. cepacia from January 2015 to November 2020. Clinical specimens of all the indoor and outdoor patients of all age groups, from whom B. cepacia was isolated, were included in the study. Identification and antimicrobial susceptibility testing of isolates were done by the Vitek 2 Compact system as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results
Ninety-one (0.54%) B. cepacia were isolated out of 16 840 organisms from 45 743 specimens received during the duration of the study. These were isolated most commonly from patients in the 0–20 years age group (31%) followed by those 41–60 years of age (20%). Also it was isolated more often in males than females. Blood and body fluids (57%) were the most common specimens from which B. cepacia was isolated followed by respiratory specimens (18%), urine (14%), and pus (11%). B. cepacia antimicrobial resistance was seen more commonly to ticarcillin–clavulanate (72%) followed by levofloxacin (34%), trimethoprim– sulphamethoxazole (30%), ceftazidime (30%), minocycline (21%) and meropenem (14%).
Conclusion
The prevalence of B. cepacia was low. B. cepacia has been identified as an important pathogen in bloodstream infections. It is important to know the antimicrobial resistance pattern of B. cepacia for better management of patients.
INTRODUCTION
Burkholderia cepacia (B. cepacia) is the fourth most common pathogenic non-fermenting gram-negative bacillus (NFGNB) isolated from clinical samples in hospitalized patients.1 It is found ubiquitously in soil, water, fruits, and vegetables.2–4 It is an emerging opportunistic pathogen causing a wide range of infections in immunocompromised and hospitalized patients having bacteraemia, particularly in patients with indwelling catheters, urinary tract infections, septic arthritis, peritonitis, and respiratory tract infections.1,2,5
B. cepacia survives and multiplies in the aqueous hospital environment for a prolonged period. There are many nosocomial outbreaks reported in the literature due to contaminated disinfectants, distilled water, 0.5% chlorhexidine solution, nebulizer solution, medical devices, and intravenous solution.2,3
B. cepacia shows a high level of intrinsic resistance to commonly used antimicrobial agents.2 Many times, this organism is reported as NFGNB due to a lack of awareness and difficulties in identification by microbiology laboratories.1 We did this study to ascertain the prevalence and antimicrobial resistance pattern of B. cepacia at a tertiary care teaching hospital.
METHODS
We did a retrospective observational study from January 2015 to November 2020 at our tertiary care teaching hospital after approval by the Institutional Ethics Committee. All specimens such as urine, respiratory samples (sputum, endotracheal and tracheal aspirate, and broncho-alveolar lavage), pus, blood, and sterile body fluids (pleural fluid, cerebrospinal fluid, ascitic fluid) received for culture and antimicrobial susceptibility test were included in the study. The samples were processed in the microbiology laboratory of a central diagnostic laboratory which is accredited by National Accreditation Board of Laboratories for Testing and Calibration. The specimens were processed as per the standard protocol. Conventional culture methods were used and the isolates were processed for identification and antimicrobial susceptibility tests by the Vitek 2 Compact system (BioMerieux, Marcy l’Etoile, France) as per the Clinical and Laboratory Standards Institute (CLSI) guidelines. The antimicrobials tested were ticarcillin–clavulanate, ceftazidime, meropenem, minocycline, levofloxacin, and trimethoprim–sulphamethoxazole. MIC50 and MIC90 were calculated for each antimicrobial after arranging all MIC values in ascending order. The patient’s demographic details, location in the hospital, associated comorbid conditions, and clinical diagnosis were collected from the laboratory and hospital information system. Duplicate isolates from a patient from the same specimen were excluded. Data collected was analyzed using Microsoft Excel 2010.
RESULTS
Ninety-one (0.54%) B. cepacia were isolated out of 16 840 organisms from 45 743 specimens received during the study duration. The most common patient age group was 0–20 years (31%) followed by 41–60 years (20%). Males (n=61) were more than females (n=30). Fig. 1 shows the year-wise prevalence, which remained below 1% and did not show an increasing trend. Blood and body fluids (57%) were the most common specimens from which B. cepacia was isolated followed by respiratory specimens (18%), urine (14%), and pus (11%). B. cepacia was isolated more from clinical specimens received from wards followed by those from the medical intensive care unit (Table I). Of the 91, comorbid conditions were seen in 45 isolates of B. cepacia. The most common comorbid conditions were chronic kidney disease (26%) followed by hypertension (24%), chronic obstructive pulmonary disease (17%), diabetes mellitus (13%), and malignancy (6%).
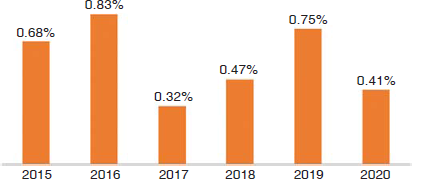
- Year-wise prevalence of Burkholderia cepacia (n=91)
| Location | n (%) |
|---|---|
| Ward | 48 (52.7) |
| Trauma | 16 (33) |
| Male and Female medical | 9 (19) |
| Surgery | 9 (19) |
| Chest medicine | 5 (10) |
| Obstetrics and gynaecology | 4 (8) |
| Privilege | 3 (6) |
| Orthopaedics | 1 (2) |
| Paediatrics | 1 (2) |
| Intensive care unit | 39 (42.8) |
| Medical | 16 (18) |
| Paediatric | 10 (11) |
| Surgical | 9 (10) |
| Cardiac | 4 (4.5) |
| Nephrology unit | 4 (4.5) |
B. cepacia antimicrobial resistance was seen more commonly to ticarcillin–clavulanate (72%) followed by levofloxacin (34%), trimethoprim–sulphamethoxazole (30%), ceftazidime (30%), minocycline (21%), and meropenem (14%). The year-wise resistance to the above antimicrobials is shown in Table II.
| Antibiotic | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Ticarcillin–clavulanate | 85 | 62 | 40 | 80 | 63 | 100 |
| Levofloxacin | 29 | 52 | 23 | 30 | 19 | 5 0 |
| Trimethoprim–sulphamethoxazole | 29 | 52 | 35 | 33 | 17 | 1 3 |
| Ceftazidime | 29 | 29 | 29 | 40 | 10 | 4 3 |
| Minocycline | 14 | 24 | 42 | 42 | 5 | 0 |
| Meropenem | 14 | 10 | 0 | 18 | 10 | 2 9 |
Resistance to ticarcillin–clavulanate, ceftazidime, meropenem, and levofloxacin increased while resistance to co-trimoxazole and minocycline decreased. B. cepacia showed susceptibility towards various antimicrobials. (Table III).
| Antimicrobial agent | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|
| Ticarcillin–clavulanate | <14 | >128 |
| Levofloxacin | 2 | >8 |
| Trimethoprim–sulphamethoxazole | <20 | 160 |
| Ceftazidime | 8 | >32 |
| Minocycline | 4 | >16 |
| Meropenem | 4 | >16 |
DISCUSSION
Pseudomonas aeruginosa and Acinetobacter spp. have been responsible for more than 80% of hospital-acquired infections till the emergence of newer NFGNB such as B. cepacia, Aeromonas, Crysobacterium, and Stenotrophomonas maltophilia.5–7 B. cepacia is non-pathogenic in healthy hosts. It is commonly associated with colonization and pulmonary infection in patients with cystic fibrosis and chronic granulomatous illness. Once patients are infected, it is difficult to eradicate because of intrinsic resistance to commonly used antimicrobials and commonly used disinfectants in hospitals.8 Infections caused by B. cepacia include pneumonia, bacteraemia, skin and soft tissue infections, and genitourinary tract infections secondary to urethral instrumentation. Many outbreaks have been reported in the literature by solutions (such as antiseptics, disinfectants, and nebulizer solutions) contaminated with B. cepacia in hospitalized patients.1,2 Thus, knowledge of the epidemiology and antimicrobial susceptibility patterns allows for the development of empirical therapeutic strategies. While there many studies on Pseudomonas and Acinetobacter spp.; there is limited data for B. cepacia. Its high transmissibility between hospitalized patients and resistance to antimicrobials makes it an organism of concern.
Prevalence of NFGNB varies between communities, hospitals, and among different patient populations in healthcare facilities. We found the prevalence of B. cepacia was 0.54% over nearly 6 years. Published studies report the prevalence of B. cepacia varies from 0.05% to 69.1%.5,8,9,10 Srinivasan et al. and Chawla et al. concluded that infections caused by B. cepacia were hospital-acquired and associated with risk factors such as insertion of intravenous line, central line, tracheostomy, Foley catheter, and hospital stay of 9–10 days.8,9 Demirdag et al. in a study done at a tertiary children’s care hospital found a high prevalence of 94.6% with children having underlying diseases such as neuromotor disorders, cerebral palsy, malignancies, cystic fibrosis, and a history of prior hospital admission.10 The year-wise prevalence during our study did not show any major changes in isolation of B. cepacia. Gautam et al. found an increased isolation rate of B. cepacia in children admitted in the Advanced Paediatric Centre than other wards of Postgraduate Institute of Medical Education and Research, Chandigarh.11 Mohankumar et al. found 41% isolates in those >60 years of age followed by 40–60 years age group.1 This is at variance with our finding where the commonest age group of patients was ≤20 years. Abdelfattah et al. found an epidemiological outbreak of B. cepacia was due to the use of contaminated ultrasound probe gel while insertion of a central venous catheter and the median age involved was 52 years.12
Location-wise distribution is also important as a part of the analysis of isolates of B. cepacia. It may help from an infection control point of view to prevent outbreaks and pseudo-outbreaks due to disinfectants and anaesthetic solutions contaminated by B. cepacia. The locations in our study were 52.7% in wards followed by 42.8% in ICUs; similar to the study by Demirdag et al. in which the distribution of patients was more from medical wards than other locations in the hospital.10 However, other studies found isolates of B. cepacia to be more in ICU patients who had more invasive procedures.1,9,13 Bacteraemia caused by B. cepacia is most often associated with polymicrobial catheter-related infection and it has been reported in patients with cancer and those undergoing haemodialysis.1,7 In our study, 57.1% of B. cepacia were from blood and body fluids culture followed by 17.5%, 14.2%, and 10.9% from respiratory, urine, and pus specimens respectively which is similar to other studies.1,5,9,10 Diabetes, renal failure, malignancy, hepatic failure, urinary catheter, mechanical ventilation, central venous catheter/ intravenous cannula, prolonged hospital stay, prolonged ICU stay, prior hospitalization, and prior antimicrobial use were risk factors and predisposing factors in patients with isolates of B. cepacia.1,7,10 In our study, the most common comorbid condition was chronic kidney disease followed by hypertension, chronic obstructive pulmonary disease, diabetes mellitus, and malignancy. However, we could not study the associated risk factors and duration of hospitalization.
B. cepacia showed an intrinsic resistance to ampicillin, amoxicillin, piperacillin, ticarcillin, ampicillin–sulbactam, amoxicillin–clavulanate, ertapenem, polymyxin B, colistin and fosfomycin.14 Ceftazidime, minocycline, meropenem, and cotrimoxazole are the drugs of choice in infection with B. cepacia.2,15 Ceftazidime, piperacillin–tazobactam, and meropenem should be used as effective antimicrobials for B. cepacia grown from blood cultures.7 The mechanisms responsible for antimicrobial resistance are an impermeable selective outer membrane, an efflux pump mechanism, and/or the production of an inducible chromosomal beta-lactamase as PenB and PenR (AmpR) system.9,16
In our study, maximum resistance was against ticarcillin– clavulanate (72%) followed by levofloxacin (32%), co-trimoxazole (30%) and ceftazidime (30%). In a systematic review by Avgeri et al., there were higher susceptibility rates of B. cepacia to imipenem, quinolones, trimethoprim/sulphamethoxazole, and third-generation cephalosporins.15 In another study, the highest susceptibility of isolates to co-trimoxazole was followed by levofloxacin and minocycline.17 Similar to these findings, trimethoprim/sulphamethoxazole were more susceptible than meropenem, ceftazidime, levofloxacin, and piperacillin/ tazobactam in other studies.5,9,12 Shah et al. found isolates were 100% susceptible to meropenem and 50% susceptible to levofloxacin, co-trimoxazole, and minocycline.18 Similar to our findings, susceptibility to meropenem varied from 60% at the start, increased to 70% by mid-study, and decreased to 43% at the end of the study. In contrast to our findings, minocycline showed a decrease in susceptibility from 100% to 74%. Cotrimoxazole susceptibility varied from 80% to 89% and ceftazidime susceptibility varied from 83% to 65%.17 Combinations of microbial agents such as meropenem with ciprofloxacin and tobramycin as well as ceftazidime–tobramycin were reported successful in treating B. cepacia.2
This being a retrospective study, we reported all isolates with clinical correlation but could not study the possible source of infection. However, clinicians were informed about possible sources and took more precautions in patients with comorbid conditions and immunosuppression.
Conclusions
Prevalence of B. cepacia was 0.54%. B. cepacia is an important bloodstream pathogen. Half of our patients had comorbid conditions. Antimicrobial resistance was seen more commonly to ticarcillin–clavulanate and levofloxacin whereas the least resistance was seen in minocycline and meropenem.
Conflicts of interest
None declared
References
- Epidemiology of Burkholderia cepacia complex infections in a tertiary care centre. Int J Recent Trends Sci Tech. 2016;18:405-8.
- [Google Scholar]
- A sporadic outbreak of Burkholderia cepacia complex bacteremia in pediatric intensive care unit of a tertiary care hospital in coastal Karnataka, South India. Indian J Pathol Microbiol. 2016;59:197-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic investigation of Burkholderia cepacia acquisition in two pediatric intensive care units. Infect Control Hosp Epidemiol. 2003;24:707-10.
- [CrossRef] [PubMed] [Google Scholar]
- An outbreak of Burkholderia cepacia complex pseudobacteremia associated with intrinsically contaminated commercial 0.5% chlorhexidine solution. Am J Infect Control. 2015;43:266-8.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial susceptibility of uncommonly isolated nonenteric Gram-negative bacilli. Int J Antimicrob Agents. 2005;25:95-109.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance among gram-negative nonfermentative bacteria at a teaching hospital in Saudi Arabia. J Chemother. 2001;13:260-4.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for mortality in patients with Burkholderia cepacia complex bacteraemia. Scand J Infect Dis. 2011;43:792-7.
- [CrossRef] [PubMed] [Google Scholar]
- Nonfermenting gram-negative bacilli other than Pseudomonas aeruginosa and Acinetobacter spp. causing respiratory tract infections in a tertiary care center. J Glob Infect Dis. 2013;5:144-8.
- [CrossRef] [PubMed] [Google Scholar]
- Report on the newly emerging nosocomial Burkholderia cepacia in a tertiary hospital. Medical J Armed Forces India. 2016;72:S50-S53.
- [CrossRef] [PubMed] [Google Scholar]
- Major aspects of Burkholderia gladioli and Burkholderia cepacia infections in children. Pediatr Infect Dis J. 2020;39:374-8.
- [CrossRef] [PubMed] [Google Scholar]
- Burkholderia cepacia complex: Beyond pseudomonas and acinetobacter. Indian J Med Microbiol. 2011;29:4-12.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of Burkholderia cepacia bacteraemia in a tertiary care centre due to contaminated ultrasound probe gel. J Hosp Infect. 2018;98:289-94.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiological assessment of Burkholderia cepacia complex (BCC) isolates in Alexandria Main University Hospital. Alexandria J Med. 2015;51:41-6.
- [CrossRef] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing In: CLSI supplement M100 (32nd ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
- [Google Scholar]
- Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: A systematic review of the clinical evidence. Int J Antimicrob Agents. 2009;33:394-404.
- [CrossRef] [PubMed] [Google Scholar]
- Accurate identification and epidemiological characterization of Burkholderia cepacia complex: An update. Ann Clin Microbiol Antimicrob. 2019;18:7.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of persistent Burkholderia cepacia complex bacteremia with ceftazidime-avibactam. Antimicrob Agents Chemother. 2018;62:e02213-17.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and antibiotic profile of non-fermenters at tertiary care hospital. Int J Biomed Adv Res. 2018;9:316-18.
- [Google Scholar]




