Translate this page into:
Primary varicella zoster virus infection-related hemiparesis and fatal neurological complications in an immunocompetent girl
2 Department of Paediatrics, Baba Raghav Das Medical College, Gorakhpur, Uttar Pradesh, India
Corresponding Author:
Vijay P Bondre
Division of Encephalitis, ICMR-National Institute of Virology, Pune, Maharashtra
India
vpbondre@gmail.com
| How to cite this article: Deoshatwar AR, Behera SP, Kumar N, Misra BR, Deval H, Bondre VP, Mittal M. Primary varicella zoster virus infection-related hemiparesis and fatal neurological complications in an immunocompetent girl. Natl Med J India 2019;32:381-382 |
Hemiparesis associated with primary varicella zoster virus (VZV) before eruption of rashes and within 10 days of infection has not been reported so far. VZV is sustained in the population almost exclusively through human-to-human transmission.[1] Although the Indian Academy of Pediatrics (IAP) recommends dual VZV vaccination at 15 months and at 4–6 years of age,[2] uptake of the vaccine through private healthcare providers is low. It has been estimated that 0.01%–0.25% of patients with VZV develop neurological complications.[3] Almost half of childhood hemiparesis has been associated with VZV. The middle cerebral artery (MCA) is reported to be involved in a majority of cases.[4],[5],[6],[7]
An 8-year-old immunocompetent girl from a poor socioeconomic background presented to our tertiary care hospital with fever, rashes and hemiparesis. The patient presented to the emergency department on 27 November 2016, with a history of fever since 21 November. The patient developed malaise with slight weakness on the right side on 22 November. On 23 November, she developed sparse rashes over the face and abdomen. On 24 November, she developed headache, neck pain and high-grade fever, followed by right-sided hemiparesis with deviation of the right angle of the mouth; she was unable to talk (dysarthria) and walk and she had altered sensorium. There was no history suggestive of an immune-compromised state. She had a pulse rate of 76/minute, respiratory rate of 32/minute, blood pressure 90/70 mmHg and weighed 25 kg. No pallor, icterus, clubbing, pedal oedema, facial puffiness or lymphadenopathy was present. The Glasgow coma scale (GCS) score was 11 at admission, which became 7 on day 3 of hospitalization. Both pupils were semi-constricted and reacting sluggishly to light, neck rigidity was absent, muscle tone was slightly increased bilaterally, deep tendon reflexes were normal, superficial abdominal reflexes were absent and plantar reflexes were recorded as bilaterally extensor (which may be due to irritation of the central nervous system). She had weakness on the right side with power of both the right upper and lower limb 2+, whereas the left side had normal 5+ power.
We followed the guidelines of the IAP for the treatment of patients with acute encephalitis syndrome[8] and started with paracetamol, azithromycin, phenytoin, ranitidine, acyclovir, vitamin B, vitamin A and vitamin K. The patient was put on synchronized intermittent mechanical ventilation (SIMV). Her investigations revealed a haemoglobin of 10.8 g/dl, total leucocyte count of 5100/cmm and a platelet count of 263 000/cmm. Her blood urea at admission was 64.5 mg/dl with a serum creatinine of 0.72 mg/dl. Her aspartate aminotransferase and alanine aminotransferase levels were normal. Her cerebrospinal fluid (CSF) examination was non-contributory (normal pressure, clear fluid, leucocyte count 4 cells/cmm, glucose 114 mg/dl, protein 35.2 mg/dl). The CSF analysis was not suggestive of bacterial infection. Her arterial blood gas values showed hypoxaemia [Table - 1]. On day 4 of admission, her condition suddenly deteriorated and she died. The cause for deterioration was not apparent. Her rapid diagnostic tests for HIV I/II antigen, hepatitis B surface antigen, Salmonella typhi IgM and IgG and malaria antigen were negative.
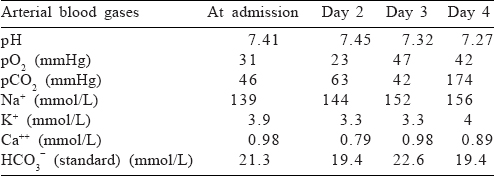
Serological and genomic assays used for the diagnosis of encephalitic viral/bacterial infections tested negative, except the anti-VZV IgM antibody (serum as well as CSF) and VZV polymerase chain reaction (fluid and scrapings from the lesions).[9] Although computed tomography (CT) scan is recommended before lumbar puncture (LP) in a patient with altered sensorium, it is delayed in patients with clinical signs of raised intracranial pressure (ICP). This patient had no papilloedema or other signs of raised ICP and she could not be shifted to another centre as she could have required ventilation support. Hence, an LP was performed as per the protocol on the day of admission (day 1).
Primary VZV infection or reactivation of the virus can cause haemorrhagic/vasculopathic complications in cerebral tissue.[3],[4],[5],[6],[8] Symptoms such as hemiparesis and deviation of angle of mouth on the same side are indicative of MCA stroke-like pathology, which has been reported in many cases.[5] The patient was put on SIMV immediately after admission; the GCS scores did not improve subsequently and hence she could not be taken for magnetic resonance imaging or CT assessment. The absence of this information remains a limitation of this report.
VZV virus can cause hemiparesis/hemiplegia and a rapidly progressive fatal complication. In a case of otherwise unexplained childhood hemiparesis/hemiplegia, a suspicion of VZV infection and subsequent treatment may prove life-saving. Vaccination against VZV during childhood and/or before visiting endemic regions is advisable.
Conflicts of interest. None declared
| 1. | Arwin AM, Gilden D. Varicella-zoster virus. In: Knipe DM, Howley PM (eds). Fields virology. 6th ed. Philadelphia:Wolters Kluwer Health/Lippincott Williams; 2013:2015–57. [Google Scholar] |
| 2. | IAP recommended 2016 vaccination schedule. Available at www.iapindia.org/ page.php?id=129 (accessed on 8 Mar 2017). [Google Scholar] |
| 3. | Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: Diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009;8:731–40. [Google Scholar] |
| 4. | Miravet E, Danchaivijitr N, Basu H, Saunders DE, Ganesan V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol 2007;49:417–22. [Google Scholar] |
| 5. | Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella-zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology 2013;80:62–8. [Google Scholar] |
| 6. | Sebag O, Mas JC, Bebin B, Ferracci JP, Sebag F. Leukoencephalitis with hemiplegia during chickenpox. Arch Pediatr 1997;4:1100–2. [Google Scholar] |
| 7. | Hansen LM, Sloth-Fjordside L, Dunkhase-Heinl U. Acute hemiparesis after chickenpox. Ugeskr Laeger 2006;168:2261–2. [Google Scholar] |
| 8. | Sharma S, Mishra D, Aneja S, Kumar R, Jain A, Vashishtha VM, et al. Consensus guidelines on evaluation and management of suspected acute viral encephalitis in children in India. Indian Pediatr 2012;49:897–910. [Google Scholar] |
| 9. | Olsen SJ, Campbell AP, Supawat K, Liamsuwan S, Chotpitayasunondh T, Laptikulthum S, et al. Infectious causes of encephalitis and meningoencephalitis in Thailand, 2003–2005. Emerg Infect Dis 2015;21:280–9. [Google Scholar] |
Fulltext Views
2,154
PDF downloads
933




