Translate this page into:
Pseudo-subarachnoid haemorrhage: An unusual presentation of hyperviscosity syndrome
Correspondence to HYMA JOSE; hymajoset@gmail.com Kochupurackal (H), Ruby Nagar (P.O.), Changanacherry, Kottayam 686103, Kerala, India
[To cite: Thomas A, Jose H, Jacob L, Koshy JM, Sebastian GM. Pseudo-subarachnoid haemorrhage: An unusual presentation of hyperviscosity syndrome. Natl Med J India 2024;37:30–1. DOI: 10.25259/NMJI_336_20]
Abstract
Hyperviscosity syndrome can present with haematological, neurological or cardiovascular manifestations. The common differential diagnoses for severe headache and altered sensorium in a patient with Eisenmenger syndrome include brain abscess, meningitis, cortical venous thrombosis and subarachnoid haemorrhage (SAH). We report a patient with Eisenmenger syndrome with hyperviscosity, presenting as pseudo-SAH, which was successfully treated with phlebotomy.
INTRODUCTION
Hyperviscosity syndrome refers to a constellation of symptoms caused by an increase in blood viscosity. The causes include paraproteinaemia, myeloproliferative neoplasms, cryoglobulinaemia, immunoglobulin G4 disease and congenital cyanotic heart diseases.1 The common symptoms are headache, dizziness, visual disturbances, mucosal haemorrhages and high-output cardiac failure.1 We report a 56-year-old woman with congenital heart disease, who presented with severe headache and altered sensorium. Her computed tomography (CT) scan mimicked extensive subarachnoid haemorrhage (SAH). To the best of our knowledge, there are no reported cases of hyperviscosity syndrome presenting as pseudo-SAH in a patient with Eisenmenger syndrome.
THE CASE
A 56-year-old woman, a known case of ventricular septal defect with Eisenmenger syndrome, was referred to our hospital as a case of SAH. She had a history of headache, which began 2 weeks back, gradually increasing in severity, leading to altered sensorium and decreased responsiveness. She was initially taken to a private hospital, where a non-contrast CT scan revealed SAH. However, a CT angiogram showed no evidence of arteriovenous malformation or aneurysm. Despite a poor Glasgow coma score (GCS), she was deemed unfit for surgery in view of her severe cardiac ailment and was subsequently referred to our hospital. On examination, she had a GCS of 7/15. General examination revealed conjunctival hyperaemia, periorbital oedema, central cyanosis and grade 3 clubbing. Her blood pressure was 120/60 mmHg, pulse 82/minute, SpO2 58% on room air and 84% with non-invasive ventilation and respiratory rate 28/minute. Her jugular venous pressure was elevated with prominent a and v waves. Apical impulse was localized over the right fifth intercostal space lateral to the midclavicular line. She had bilateral basal crackles. A non-contrast CT scan of the brain was repeated, which showed extensive SAH involving bilateral cerebral hemispheres and basal cisterns with diffuse brain oedema, mild effacement of the lateral ventricle and mass effect on the midbrain with mild foramen magnum herniation (Figs 1a,b). X-ray chest showed dextrocardia with bilateral lower zone non-homogenous opacities (Fig. 2). Her haemoglobin was 22.9 g/dl, packed cell volume (PCV) 73.8, total leucocyte count 7200/cmm with 85% polymorphs and platelet count 43 000/cmm. Renal function tests and electrolytes were within normal limits. Serum iron was 84 µg/dl, serum ferritin 250 ng/ml and uric acid 6.85 mg/dl. Liver function tests were normal except for mildly elevated aspartate aminotransferase (55 i.u./L) and alkaline phosphatase (145 i.u./L). Activated partial thromboplastin time was 89 seconds and international normalized ratio (INR) was 6.35. She was provisionally diagnosed as having secondary polycythaemia-hyperviscosity syndrome and was initiated on intravenous fluids, which was titrated based on collapsibility of the inferior vena cava and extent of b lines on lung ultrasonogram. Phlebotomy (300 ml) was done and an equal volume of fresh frozen plasma was replaced. Her sensorium gradually improved and INR dropped to 1.81. Haemoglobin was 20.8 g/dl and PCV was 65.5. Two days later, the PCV again rose to 70.3 and INR to 2.86 and another phlebotomy (200 ml) was done. Ultrasound scan of the abdomen showed situs inversus totalis. Echocardiography revealed ventricular septal defect with Eisenmenger syndrome. Her sensorium improved markedly (GCS 15/15), she was gradually weaned out of NIV support and an intake restriction of 1 L/day was followed. Platelet count improved to 150 000 lakh/cmm. One week later, she was discharged, maintaining an SpO2 of 85% on room air with normal respiratory rate.
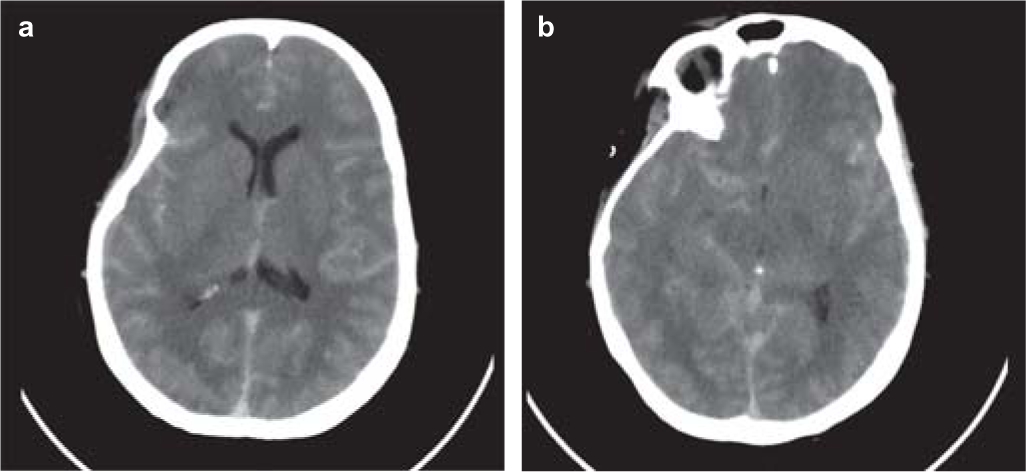
- Non-contrast computed tomography scan of the brain showing increased density of subarachnoid spaces with diffuse cerebral oedema and mild effacement of ventricles
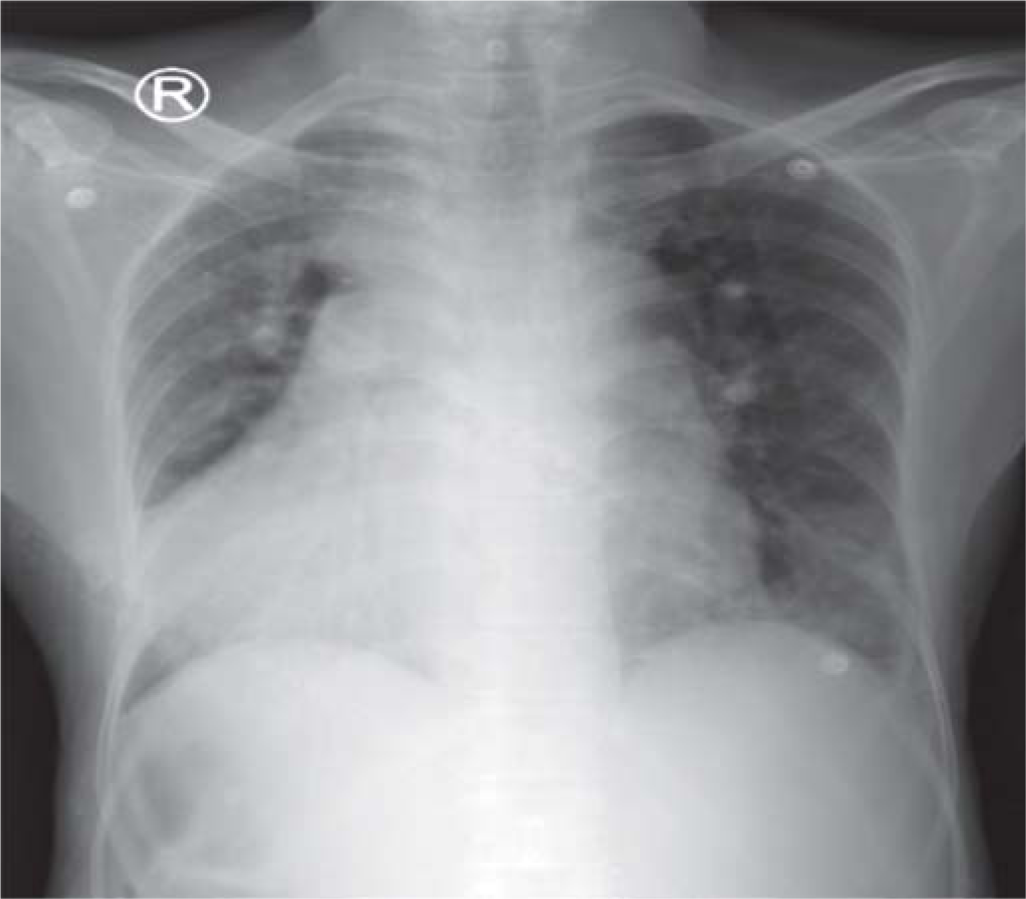
- Chest X-ray showing dextrocardia
DISCUSSION
There are two interesting aspects to this case: hyperviscosity syndrome and the resulting pseudo-SAH appearance in the CT scan. The underlying pathology was a ventricular septal defect with Eisenmenger syndrome. Eisenmenger syndrome is a multisystem disorder including hypertrophic osteoarthropathy, renal dysfunction, coagulation disorders, heart failure and premature death.2
Our patient presented with severe headache and altered sensorium and a CT scan of the brain was suggestive of SAH. SAH is characterized by increased density of the basal cisterns and subarachnoid spaces on CT scan.3 There are few conditions that have a radiological appearance mimicking SAH. Diffuse cerebral oedema, purulent meningitis, infarction, contrast extravasation, status epilepticus, spontaneous intracranial hypotension, after myelography and polycythaemia are all well-described causes of pseudo-SAH on CT scan.4–11 Therefore, a CT scan should be interpreted in the context of the clinical scenario. Our patient had gradual worsening of headache and altered sensorium in contrast to the sudden onset of severe headache that is encountered in true SAH. Poly-cythaemia may mimic SAH on non-contrast CT.10,11 Diagnosis of hyperviscosity syndrome can be made by checking the haemoglobin and haematocrit levels in a patient with hyperdense vessels on non-contrast CT.
Blood viscosity depends on multiple factors including red blood cell mass and morphology, plasma viscosity, temperature and shear stress.12 In compensated secondary erythrocytosis (stable haemoglobin in an iron-replete state), even with haematocrit levels higher than 70%, hyperviscosity symptoms are absent or mild. Patients with decompensated erythrocytosis can complain of moderate-to-severe hyperviscosity symptoms at lower haematocrit levels.13 Our patient had severe hyperviscosity symptoms even in an iron-replete state. Iron deficiency must be avoided in patients with Eisenmenger syndrome. Phlebotomy may aggravate hyperviscosity symptoms in iron-deplete individuals.
The first step in the management of hyperviscosity syndrome is to correct dehydration, if present.2 Dehydration can further increase blood viscosity. Our patient had poor food intake for days before getting admitted in the hospital and this could have worsened her symptoms. Hence, she was started on intravenous fluids before phlebotomy was done. Ensuring optimal hydration in these patients is a challenging task. Overhydrating could aggravate the symptoms of cardiac failure and aggressive restriction of fluids can result in worsening of hyperviscosity symptoms. Our patient had a deranged INR along with thrombocytopenia, which prompted us to use fresh frozen plasma following phlebotomy. It should be noted that INR could spuriously be deranged in patients with polycythaemia if standard citrate vials are used. In case of mild symptoms, serum iron levels should be checked and iron supplements added, if found to be deficient. There are two indications for phlebotomy: (i) moderate-to-severe hyperviscosity symptoms due to secondary erythrocytosis and (ii) preoperative phlebotomy for autologous blood donation if the haematocrit level is >65%.2 Hyperviscosity will disappear after adequate phlebotomy within 24 hours resulting in increased cardiac output and systemic blood flow.
Clinicians should be aware of this rare presentation as failure to recognize hyperviscosity syndrome and pseudo-SAH can result in the institution of unnecessary medical and surgical interventions and increase morbidity.
Conflicts of interest
None declared
References
- Acute hyperviscosity: Syndromes and management. Blood. 2018;132:1379-85.
- [CrossRef] [PubMed] [Google Scholar]
- The adult patient with Eisenmenger syndrome: A medical update after Dana point part III: Specific management and surgical aspects. Curr Cardiol Rev. 2010;6:363-72.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage: A potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003;24:254-6.
- [Google Scholar]
- Acute purulent leptomeningitis mimicking subarachnoid hemorrhage on CT. J Comput Assist Tomogr. 1994;18:126-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage in cerebellar infarction. Neurocrit Care. 2007;7:172-4.
- [CrossRef] [PubMed] [Google Scholar]
- False positive appearance of subarachnoid hemorrhage on CT with bilateral subdural hematomas. Radiat Med. 1999;17:439-42.
- [Google Scholar]
- Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol Sci. 2012;33:445-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage: A CT-finding in spontaneous intracranial hypotension. Neurology. 2005;65:135-7.
- [CrossRef] [PubMed] [Google Scholar]
- Unique features of polycythemia observed on plain non contrast CT scan of head. J Pediatr Neurosci. 2010;5:27-9.
- [CrossRef] [PubMed] [Google Scholar]
- Blood viscosity and its relationship to iron deficiency, symptoms, and exercise capacity in adults with cyanotic congenital heart disease. J Am Coll Cardiol. 2006;48:356-65.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic hypoxaemia and decompensated erythrocytosis in cyanotic congenital heart disease. Lancet. 1986;2:313-15.
- [CrossRef] [PubMed] [Google Scholar]




