Translate this page into:
Quality of informed consent in cancer clinical trials in India: A cross-sectional survey
2 Public Health Research Centre, New York University, Abu Dhabi, United Arab Emirates
Corresponding Author:
Vikram Gota
Department of Clinical Pharmacology, Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Sector 22, Kharghar, Mumbai 410210, Maharashtra
India
vikramgota@gmail.com
| How to cite this article: Gota V, Nookala M, Yadav A, Menezes SR, Kannan S, Ali R. Quality of informed consent in cancer clinical trials in India: A cross-sectional survey. Natl Med J India 2018;31:334-338 |
Abstract
Background. An ‘informed consent’ is a legal and ethical requirement for research involving human subjects. Studies assessing the validity of informed consent and determinants of its quality have highlighted problems in consent delivery and comprehension by trial participants. We report the findings of a questionnaire-based survey conducted to understand the quality of informed consent (QuIC) in cancer clinical trials.Methods. The survey was conducted in a single tertiary care cancer centre in India. Patients enrolled in phase 1, 2 or 3 interventional studies were administered the QuIC questionnaire by a trained study coordinator. The QuIC, expressed as knowledge score, was calculated from the proportion of correct responses expressed as a percentage.
Results. The mean (SD) knowledge score was 60.46% (1 5.21%). It was considerably higher in industry-sponsored trials compared to investigator-initiated trials (65.32% v. 52.21%, respectively; p<0.001). Faith in the treating oncologist positively influenced the patients’ decision to participate in a trial. Nearly 97% of the respondents anticipated better care, while 85% felt that the new drug/procedure would be better than the existing treatment. Free treatment emerged as a strong inducement for patients to take part in clinical trials. Patients were aware of their autonomy, and responses showed that none of the patients were coerced or unduly influenced to participate in clinical trials.
Conclusion. Our study revealed important deficiencies in research participants’ understanding of essential elements of informed consent. Thorough patient counselling is crucial to minimize ‘therapeutic misconception’ to preserve the validity of informed consent in cancer trials.
Introduction
The communication that takes place between patients and their physician resulting in the patient’s authorization to undergo a specific medical intervention is called informed consent. It is more than just a legal prerequisite; it is a moral and ethical requirement.[1] It protects a patient’s right to shared decision-making. While obtaining consent from patients is important in day-to-day medical practice, informed consent in research involving humans is clothed in an additional layer of complexity, often due to the investigational nature of the intervention. However, published studies on clinical trial participants in the USA reveal that the informed consent obtained, even from individuals with no cognitive impairments, is not always valid.[2] For example, in a hypertension study based in Washington D.C., one-third of the patients did not understand the trial’s double-blind design a few minutes after giving their consent. Furthermore, less than a third of them were able to recall 2 side-effects of the study drug.[3] Other studies in the West have found that patients’ understanding of the purpose of research ranges from 80% to just 10%.[4]-[5] Thus, the ability of clinical trial participants to take part in an informed consent process may be limited. To protect the interest of study patients and the integrity of clinical research, consent obtained from each patient should be formally assessed. Components of a valid informed consent include capacity, autonomy, disclosure, understanding, voluntariness and permission. The US regulations governing research involving human subjects recommend 8 elements in informed consent.[6] The extent of information and the perception of trial patients vary considerably; patients are not cognisant of the experimental nature of clinical trials,[7],[8],[9],[10],[11],[12] while some tend to believe that clinical trials are designed for their benefit rather than the benefit of future patients.[13] This belief has been termed the ‘therapeutic misconception’. At least 20% of patients do not realize that they have the freedom to withdraw their consent.[9]
Much has been written about the ethical concerns that stem from an increasing number of clinical trials conducted in India and the lack of sufficient regulatory oversight.[14],[15] As a large percentage of India’s patient population is illiterate or unacquainted with medical research, it is more difficult to ensure that patients who agree to take part in trials are fully cognisant of the risks and benefits of doing so. In the public sector, this gets more challenging due to paternalistic doctor–patient relationships that could lead to exploitation.[16] Poor socioeconomic conditions often result in the standard of care being unaffordable; therefore, free treatment offered in clinical trials could prove to be a huge incentive to participate.[17] Although these challenges are not exclusive to India, we did a survey of clinical trial participants at a tertiary oncology centre to better understand some of these issues. We aimed to assess their motives for giving consent and to evaluate how informed their consent was.
Several problems are unique to India when it comes to the process of consenting for clinical trials. Some factors that come in the way of a valid informed consent are illiteracy, vernacular language barriers, gender and age bias, social stigmas, the paternalistic relationship with doctors and lack of time due to large patient load. Tools to assess the extent of understanding of the information provided at the time of consent are relevant in the wake of latest developments in areas of compensation for trial-related injury or death and the mandatory audiovisual recording of the consent process in India. Our study aimed to assess the quality of informed consent (QuIC) among Indian patients participating in cancer clinical trials.
Methods
Participants
The survey was done at a single tertiary care cancer centre in India. Patients who were enrolled in a clinical trial were included in the survey. The trials included were phase 1, 2 or 3 interventional studies of pharmacological, surgical or radiation therapy, either sponsored by the pharmaceutical industry or initiated by investigators. All trials open for accrual were reviewed in advance to see if they met these criteria. Potential participants were identified by a clinical research coordinator who kept a watch on new enrolments in the identified studies by contacting individual trial coordinators by telephone on a regular basis. Individuals were eligible if they were aged 18 years or above and had signed a consent form for a qualified cancer trial at Tata Memorial Centre, India, within the previous 7 days. Individuals were enrolled by convenience sampling and included in this survey if they could speak English, Hindi or Marathi. Individuals who had been removed from the clinical trial within 7 days of signing consent were excluded. Enrolment took place from March 2009 to December 2011. The Human Ethics Committee of Tata Memorial Centre approved the study protocol.
Survey methods
A questionnaire was developed based on the QuIC to capture all information relevant to the process of informed consent, including the level of interest shown by the participant to understand the trial, the knowledge of the participant pertaining to the trial, the people involved in the participants’ decision to enrol in the clinical study and the possible factors that encouraged the participants to give their consent. A trained clinical trial coordinator administered the questions. Written informed consent was obtained before enrolling patients in the study and patients were interviewed within 7 days of volunteering for a clinical trial. The questionnaire was administered at 2 locations of Tata Memorial Centre: Tata Memorial Hospital and the Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), the research unit of Tata Memorial Centre, which offers treatment to patients only on a research protocol.
Design of the questionnaire
The QuIC questionnaire (QICQ) consists of 4 parts. Part A consists of 3 questions that measure the level of interest shown by the participant to be informed about the study he/she has consented for (questions 2, 3 and 4). Based on the participants’ response to these questions, an interest score was calculated and categorized as ‘No interest’ (score 0), ‘Somewhat interested’ (score 1), ‘Interested’ (score 2) and ‘Most interested’ (score 3). Part B measures the knowledge of participants, consisting of 13 questions on the basic tenets of informed consent specified in the International Council for Harmonization–good clinical practice (ICH-GCP) guidelines. Questions were either categorical or open-ended. For all open-ended questions, a predetermined set of’correct’ responses were identified in accordance with the GCP. Each correct response was awarded a score of ‘1’. The proportion of right responses expressed as a percentage calculated the QuIC. The questionnaire also included questions to identify people involved in the patient’s decision to participate in the trial (Q 20–24) and the factors that encouraged them to participate in the trial (Q 25–29). Finally, we also collected demographic characteristics (e.g. age, sex and education) for each patient.
Statistical analysis
SPSS version 20 was used for statistical analysis. Bivariate associations with knowledge scores (KS) were assessed with t-tests or ANOVA. Association between categorical variables was analysed using chi-square test.
Results
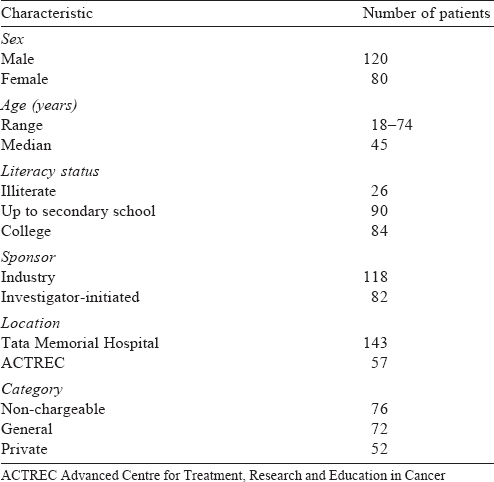
Two hundred individuals participated in our study [Table - 1] and their mean age (SD) was 44 (14.1) years. The proportion of correct answers varied greatly across individual questions of the QICQ [Table - 2]. More than half the respondents did not know what a clinical trial was (109/200) and 4 of them believed that the clinical trial they were taking part in also involved experiments in animals. One-fourth of the respondents did not agree that the main purpose of clinical trials is to benefit future patients. The vast majority (90%) were unaware that the treatment being researched was not already shown to be the best for their cancer and that the study used non-standard treatments or procedures. Seventeen per cent of the respondents did not realize that participation in the trial could involve certain risks and discomforts and 18% could not recollect at least 1 adverse effect specified in the patient information sheet. All of them felt that they would benefit in some way by participating in the trial [Table - 3]. Approximately 60% of respondents recalled being offered alternatives to their taking part, and almost all knew that they could decline participation (98%) or withdraw from the trial (92%).
The mean KS was 60.46% (15.21 %) [Figure - 1]. There was a good correlation between KS and the level of interest (p<0.001 ; [Figure - 2]. KS was significantly higher in pharmaceutical industry-sponsored trials compared to investigator-initiated trials (65.3% v. 52.2%, respectively; p<0.001) and in the trials conducted in the hospital as against the research centre (61.7% v. 56.3%, respectively; p=0.02; Figure 3]). Although better education was associated with a higher level of interest (p=0.036), the KS were not significantly different between illiterate patients, non-college educated and college-educated groups (56.8 [18.6], 61.0 [14.8] and 61.1 [14.6], respectively).

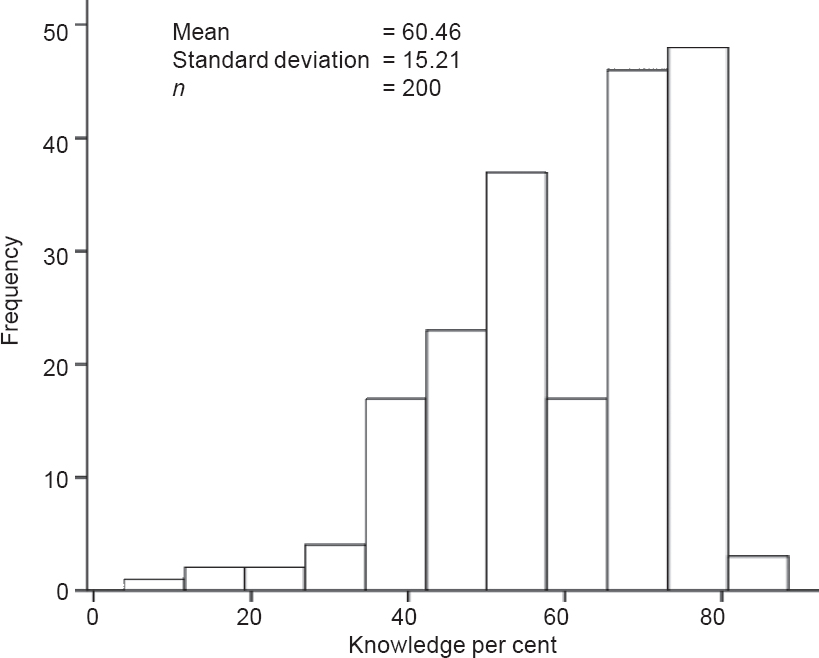 |
| Figure 1: Frequency distribution of knowledge scores of participants |
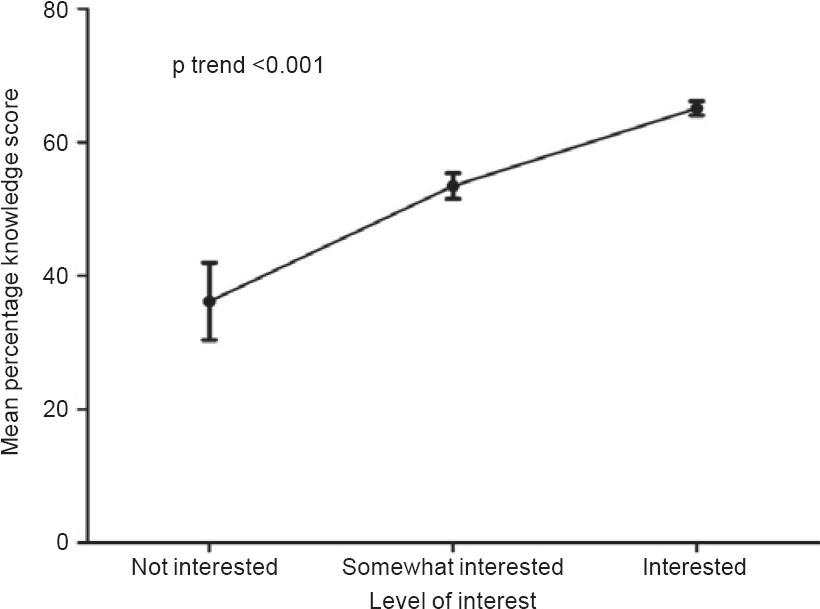 |
| Figure 2: Correlation between knowledge score (mean±standard error) and level of interest |
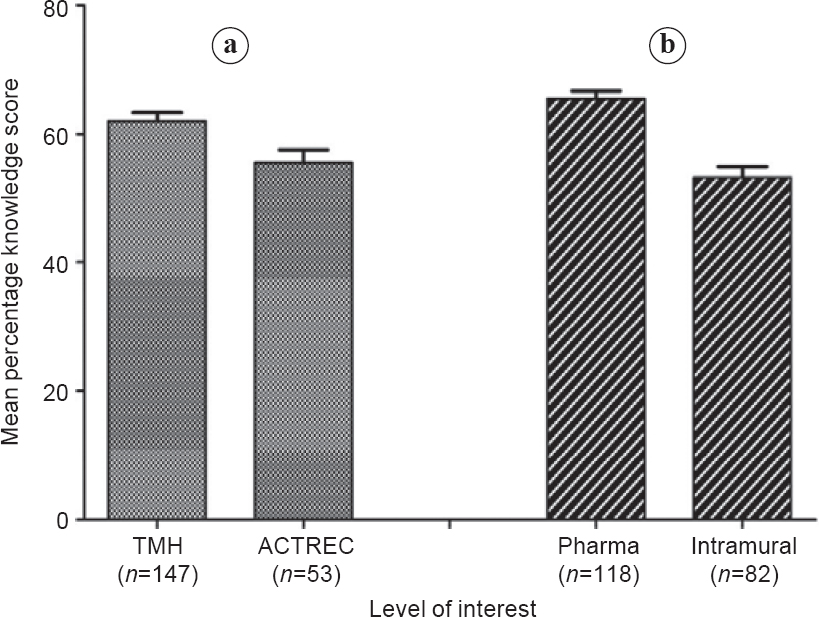 |
| Figure 3: Knowledge score (mean±standard error) of patients in different settings: (a) hospital v. research centre and (b) pharma-sponsored v. investigator-initiated trial TMH Tata Memorial Hospital ACTREC Advanced Centre for Treatment, Research and Education |
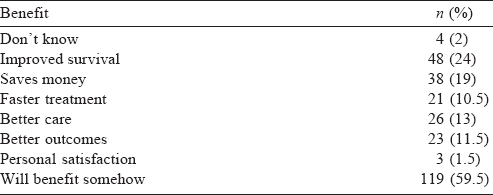
Family members played a major role in the decision of the participants to take part in a trial. In 97% of cases, at least 1 family member was involved in making the decision. Almost all of them participated in the trial because of trust in the oncologist ( 199/200), while none of them were unduly influenced to participate. A fifth consulted their personal physician whereas friends and other patients were consulted by 18% of respondents. Among the factors influencing participation in clinical trials, 97% of respondents anticipated better care, while 85% felt that the new drug/procedure would be better than the existing treatment [Table - 4].

Discussion
We investigated informed consent in clinical trials of cancer, using a questionnaire that was designed to assess the essential elements required by the ICH-GCP. The mean KS was about 60%. More than half of the respondents were unaware of the true objective and purpose of a clinical trial and 90% were not aware that non-standard treatments or procedures were being evaluated. All of them anticipated some personal benefit by participating in the trial. Knowledge varied widely and there were some crucial lapses in understanding. Major deficiencies included being unaware of non-standard treatment, the potential for additional risk or discomfort, the experimental nature of treatment and the uncertainty of benefits to themselves. These problems characterize what Appelbaum et al. referred to as ‘the therapeutic misconception’.[18] However, none of them reported being coerced into consenting and almost all of them were aware of their autonomy to withdraw their consent at any time during the study. Family played a significant role in helping the potential trial participants with their decision. However, trust in the oncologist was the single most important factor influencing their decision to consent. Family physician and friends were involved to a much lesser extent.
KS correlated well with the level of interest shown by the participant to understand the contents of the patient information sheet. These results indicate that efforts are needed to make the consent process more informative. The use of audiovisual aids and other interactive ways of imparting information about the trial may help in enhancing the QuIC.[19],[20] Patients participating in pharmaceutical industry-sponsored trials had higher KS, indicating better recall than those taking part in investigator-initiated trials. This could be due to more elaborate informed consent forms in pharmaceutical industry-sponsored studies, thereby leading to a lengthier informed consent process. Similarly, patients who were participating in trials at Tata Memorial Hospital had better QuIC scores compared to those at ACTREC. However, this difference in the QuIC based on location of the study (Tata Memorial Hospital v. ACTREC) was lost when analysis was adjusted for the type of study (pharmaceutical industry-sponsored v. investigator- initiated trial). Tata Memorial Hospital had a higher proportion of pharmaceutical industry-sponsored studies as compared to ACTREC which confounded the results. Contrary to the findings by Joffe et al.,[1],[21] KS were not associated with the literary status of the individual, although better educated candidates showed more interest in the informed consent process. Perhaps, the study was underpowered to detect a difference in KS between the literacy strata. Contrary to expectations, many factors including age and gender did not show any correlation with KS.
Although 74% claimed that altruism was one of the reasons for participation, all respondents anticipated some benefit from participating in the trials. Not surprisingly, about 60% of respondents were attracted by the free treatment being offered in trials. With a mere 15% of Indian patients being covered by health insurance, free treatment is a powerful inducement for clinical trial enrolment. Thus, the basic tenet of ‘no inducement’ adopted by GCP guidelines is challenged even by the legitimate practice of providing free treatment on trials. Some commentators have even questioned the practice of providing free treatment to trial participants in open-label trials where patients are randomized to the standard arm.[22] Such issues assume importance in economically backward countries and are topics of much debate. However, free treatment is not the only incentive, patients perceive the care provided in clinical trials to be superior to routine care. This could range from hospital beds being made available for admission, intense monitoring, close follow-up, etc. So much so that 97% of respondents participated with the hope that by doing so they would receive better care, and 70% agreed that they felt the advantages of participating in a trial were not worth foregoing. To an extent, this could be attributed to the manner in which patients were counselled by the people designated to administer informed consent. Nearly 85% of respondents were optimistic about the newer intervention and believed that it would emerge superior to the existing treatment. Thus, patients carry several misconceptions about clinical trials, which should be addressed while counselling them during the consent process. The validity of informed consent is questionable when therapeutic equipoise is distorted and trial patients have undue expectations from the test intervention.[23] The investigators and their team should take special care to clarify these doubts to ensure that patients do not participate in clinical trials for the wrong reasons. Since the goals of advancing science or treatment could conflict with the interests of present patients,[24] it is important that investigators should appreciate this conflict and help participants distinguish research goals from therapeutic intentions.[25]
Although our results suggest the need for improvement in informed consent, 2 positives emerged from this study—patients were aware of their autonomy and none of the patients were coerced or unduly influenced to participate in trials. One problem perhaps unique to our cultural context is that patients tend to consent for clinical trials solely because of faith in their oncologist. This could allow possible ‘exploitation’ of patients, especially if the oncologist, although meaning well for the patient, has a greater inclination towards the research goals. This is observed irrespective of the literacy or financial status of the patients. The validity of informed consent in these circumstances becomes questionable.
Some limitations to this study merit discussion. As we did not have enough respondents in each phase (phase 1—3), we were unable to draw phase-specific conclusions. Additionally, some items in the QuIC questionnaire may not be applicable to randomized controlled trials that compare 2 standard therapies. Some of the investigator-initiated trials were of that nature. Our questionnaire mainly addressed conceptual issues associated with clinical trial participation. We did not look at the template used in the patient information sheet. Joffe et al. reported better KS in participants who were given structured consent forms than those who were not.[1] We also did not consider the time taken to consent because all research participants in our hospital are not given time limits to make their decision. Several studies have reported that patients who are given more time to consider participation are more knowledgeable than those who did not.[26] We did not collect information on whether respondents read their consent forms carefully. Studies have shown that patients who read the consent forms carefully achieved higher KS than those who did not.[1],[16]
A trained clinical research coordinator administered the questionnaire. In spite of the training, differences might exist in the way the questionnaire was administered in the initial learning phase compared to the latter half of the study, which may contribute to reporting bias. Recall bias is less likely since all patients were surveyed within 7 days of consenting for a clinical trial.
We recorded critical flaws in research patients’ understanding of cancer clinical trials in which they volunteered to participate. We have, for the first time, shown that KS are proportional to the level of interest shown by the participants. Special efforts are needed during the consent process to minimize therapeutic misconception among clinical research participants. This drawback needs brainstorming to bring a fine balance between research ethics and the goal of advancing science.
Best practices
We recommend the following to achieve a better understanding of trial-related information among patients enrolling for clinical trials.
- Adopt measures to improve the level of interest shown by patients. The use of audiovisual aids and other interactive ways of imparting information about the trial may help in enhancing the QuIC.
- Educating trial patients on the importance of autonomy would minimize their dependence on oncologists to make ‘decisions’ on their behalf. While trust is an important element of a physician-patient relationship, it is essential for patients to realize that the therapeutic equipoise prevailing in a clinical trial setting implies that the patient is the best judge to make decisions regarding their participation.
Research agenda
Future studies should focus on developing educational and audiovisual aids and validating their utility. This could be done in a randomized controlled setting where these interventions are compared with the conventional informed consent process for improvement in KS.
Educational implications
It will be a challenge for investigators to underplay the role of trust and encourage trial participants to exercise their autonomy. This should be discussed at various platforms such as investigators’ meetings to identify best practices to achieve this objective.
Conflicts of interest. None declared
| 1. | Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: A new measure of understanding among research subjects. J Natl Cancer Inst 2001; 93:139-47. [Google Scholar] |
| 2. | Wendler D. Can we ensure that all research subjects give valid consent? Arch Intern Med 2004;164:2201-4. [Google Scholar] |
| 3. | Bergler JH, Pennington AC, Metcalfe M, Freis ED. Informed consent: How much does the patient understand? Clin Pharmacol Ther 1980;27:435-40. [Google Scholar] |
| 4. | Howard JM, DeMets D. How informed is informed consent? The BHAT experience. Control Clin Trials 1981;2:287-303. [Google Scholar] |
| 5. | Riecken HW, Ravich R. Informed consent to biomedical research in veterans administration hospitals. JAMA 1982;248:344-8. [Google Scholar] |
| 6. | 45 CFR 46 Code of Federal Regulations - General Requirements for Informed Consent; 2009. Available at www.hhs.gov/ohrp/regulations-and-policy/regulations/ 45-cfr-46/index.html (accessed on 1 Apr 2017). [Google Scholar] |
| 7. | Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 1995;13:1062-72. [Google Scholar] |
| 8. | Schaeffer MH, Krantz DS, Wichman A, Masur H, Reed E, Vinicky JK, et al. The impact of disease severity on the informed consent process in clinical research. Am J Med 1996;100:261-8. [Google Scholar] |
| 9. | Lynöe N, Sandlund M, Dahlqvist G, Jacobsson L. Informed consent: Study of quality of information given to participants in a clinical trial. BMJ 1991;303:610-13. [Google Scholar] |
| 10. | Simes RJ, Tattersall MH, Coates AS, Raghavan D, Solomon HJ, Smartt H, et al. Randomised comparison of procedures for obtaining informed consent in clinical trials of treatment for cancer. Br Med J (Clin Res Ed) 1986;293:1065-8. [Google Scholar] |
| 11. | Schultz AL, Pardee GP, Ensinck JW. Are research subjects really informed? West J Med 1975;123:76-80. [Google Scholar] |
| 12. | Penman DT, Holland JC, Bahna GF, Morrow G, Schmale AH, Derogatis LR, et al. Informed consent for investigational chemotherapy: Patients’ and physicians’ perceptions. J Clin Oncol 1984;2:849-55. [Google Scholar] |
| 13. | Baker R. The human radiation experiments: Final report of the President’s Advisory Committee on Human Radiation Experiments. Med Hist 1997;41:256-7. [Google Scholar] |
| 14. | Srinivasan S, Loff B. Medical research in India. Lancet 2006;367:1962-4. [Google Scholar] |
| 15. | Nundy S, Gulhati CM. A new colonialism? - Conducting clinical trials in India. N Engl J Med 2005;352:1633-6. [Google Scholar] |
| 16. | Sanwal AK, Kumar S, Sahni P, Nundy S. Informed consent in Indian patients. J R Soc Med 1996;89:196-8. [Google Scholar] |
| 17. | Srinivasan S. Ethical concerns in clinical trials in India. An investigation. Mumbai:Centre for Studies in Ethics and Rights; 2009. Available at www.environmentportal.in/files/Ethical_concerns_in_clinical_trials_in_India_An_ investigation.pdf (accessed on 1 Apr 2017). [Google Scholar] |
| 18. | Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Cent Rep 1987;17:20-4. [Google Scholar] |
| 19. | Hitchcock-Bryan S, Hoffner B, Joffe S, Parker C, Wolanski A, Bauer-Wu S, et al. Entering a clinical trial: Is it right for you? A randomized study of the clinical trials video and its impact on the informed consent process. Oncol Nurs Forum 2007;34:248. [Google Scholar] |
| 20. | Shukla AN, Daly MK, Legutko P. Informed consent for cataract surgery: Patient understanding of verbal, written, and videotaped information. J Cataract Refract Surg 2012;38:80-4. [Google Scholar] |
| 21. | Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: A cross-sectional survey. Lancet 2001;358:1772-7. [Google Scholar] |
| 22. | Kasaragod A. Health insurance in India: The need to come together for a common good. Indian J Med Ethics 2011;8:49-50. [Google Scholar] |
| 23. | Nadimpally S, Bhagianadh D. ‘The invisible’: Participant’s experiences in clinical trials. Perspect Clin Res 2017;8:5-10. [Google Scholar] |
| 24. | Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA 2000;283:2701-11. [Google Scholar] |
| 25. | Freedman B, Fuks A, Weijer C. Demarcating research and treatment: A systematic approach for the analysis of the ethics of clinical research. Clin Res 1992;40: 653-60. [Google Scholar] |
| 26. | Morrow G, Gootnick J, Schmale A. A simple technique for increasing cancer patients knowledge of informed consent to treatment. Cancer 1978;42:793-9. [Google Scholar] |
Fulltext Views
4,077
PDF downloads
2,143




