Translate this page into:
Rapid diagnosis of disseminated Mycobacterium avium complex infection mimicking metastatic malignancy using metagenomic next-generation sequencing
Correspondence to SHENJIE TANG; tangsj1106@vip.sina.com
[To cite: Liu Y, Wang J, Tang S, Pu Y. Rapid diagnosis of disseminated Mycobacterium avium complex infection mimicking metastatic malignancy using metagenomic next-generation sequencing. Natl Med J India 2025;38:16–17. DOI: 10.25259/NMJI_872_2022.]
Abstract
Disseminated non-tuberculous mycobacteria (NTM) disease, which is mainly found in immunocompromised individuals, is a rare and severe infection whose diagnosis poses a challenge to clinicians. We present a patient with disseminated NTM infection mistaken for metastatic malignancy in an otherwise healthy patient and the tortuous diagnostic process. Metagenomic next-generation sequencing (mNGS) played a critical role in the diagnosis. Further screening for anti-interferon-γ antibodies revealed that the patient had a potential immunodeficiency.
INTRODUCTION
Nontuberculous mycobacteria (NTM) infections, particularly disseminated disease, are rare but clinically important, often mimicking malignancies or autoimmune disorders. Diagnosis remains challenging due to limitations of conventional methods (e.g. low sensitivity of acid-fast staining and prolonged culture time). Metagenomic next-generation sequencing (mNGS) offers rapid, unbiased pathogen detection, proving critical in atypical presentations. Disseminated NTM infections in seemingly immunocompetent patients may indicate underlying immuno-deficiency, such as neutralizing anti-interferon-γ (IFN-γ) autoantibodies—a condition prevalent in Asian populations. Our report underscores the utility of mNGS in diagnosing ambiguous cases and emphasizes the importance of evaluating cellular immunity in unexplained disseminated infections.
THE CASE
A 67-year-old woman with a medical history of hypertension presented with a two-month history of multiple bone aches and night sweats. Five months prior to this presentation, she had been admitted to hospital with a recurrent fever (maximum temperature of 40 °C) accompanied by profuse sweating. During that hospitalization, enhanced computed tomography (CT) of her chest, abdomen and pelvis revealed multiple bone destruction in the thoracic, lumbar and sacral vertebrae and bilateral iliac crests, a few patchy shadows in both lower lungs, and enlarged mediastinal and axillary lymph nodes (Fig. 1). Metastatic malignancy was suspected. She subsequently underwent a series of tests to further investigate this suspected diagnosis. Positron emission tomography/computed tomo-graphy (PET/ CT) showed multiple superficial and deep lymph node enlargement, diffuse bone destruction of the skull, trunk and limb bones (Fig. 1), slight thickening of the gastric wall and a few patchy shadows in both lower lungs, indicating malignant metastasis. Further investigations were done to confirm the diagnosis of malignancy. Gastroscopy revealed chronic erosive gastritis. Histopathology of the right cervical lymph node aspirate showed hyperplastic lesions. A bone marrow biopsy revealed normal polyclonal plasma cells, with no abnormal expression of lymphoma immunophenotypes. No abnormality was found in the bone marrow either by flow cytometric analysis, leukaemia or leukaemia-related fusion gene detection assays, or serum immunofixation electrophoresis. Both the G and GM tests were negative. The patient was prescribed moxifloxacin, and her temperature returned to normal after approximately 1 month. Three months later, the patient underwent a sternal mass biopsy. The pathology results showed multiple areas of focal necrosis, multiple histiocytic reactions and the infiltration of lymphocytes and plasma cells, suggestive of possible inflamma-tion. Therefore, she was referred to our department and admitted for further investigations.
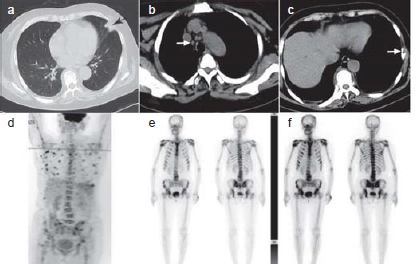
- Chest computed tomography (CT) (a) revealed patchy shadows in the left lower lung; (b) mediastinal window revealed mediastinal lymphadenopathy; (c) mediastinal window revealed multiple areas of bone destruction; (d) Positron emission tomography (PET)/CT revealed multiple areas of superficial and deep lymph node enlargement, diffuse bone destruction; (e) radionuclide bone scan revealed multiple areas of superficial and deep lymph node enlargement, diffuse bone destruction.
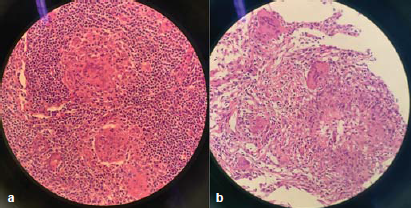
- (a) The right axillary lymph node biopsy revealed granulomatous inflammation and (b) the percutaneous pulmonary biopsy revealed granulomatous inflammation with necrosis, and multinucleated giant cell reaction.
Physical examination revealed enlarged lymph nodes under the right armpit, tenderness in the sternum. There were no other abnormalities. Laboratory tests showed an increased erythrocyte sedimentation rate (125 mm) and elevated C-reactive protein level (92.5 mg/L). Serological tests for T cell spot tuberculosis (T-SPOT.TB), human immunodeficiency virus (HIV) antibodies, syphilis antibodies, brucella antibodies and antinuclear antibody spectrum, and multiple blood cultures were all negative. Cellular immune function assays showed that the total T lymphocyte (423 cells/μl), CD4+ T lymphocyte (280 cells/μl), total B lymphocyte (156 cells/μl) and natural killer (NK) cell (123 cells/μl) counts were all decreased.
A radionuclide bone scan showed active multiple reactive bone formations (Fig. 1). The right axillary lymph node biopsy and percutaneous pulmonary biopsy revealed granulomatous inflammation with necrosis, multinucleated giant cell reaction and negative acid-fast staining. Because infection could not be ruled out, metagenomic next-generation sequencing (mNGS) was performed on the day of pulmonary biopsy. mNGS of lung biopsy specimens detected Mycobacterium (M.) intracellulare, part of the M. avium complex (MAC), confirming a diagnosis of disseminated MAC infection. A standard anti-MAC treatment regimen of 450 mg rifampicin, 600 mg amikacin, 750 mg ethambutol and 400 mg moxifloxacin daily, and 500 mg clarithromycin twice daily, was initiated. The patient’s bone pain resolved two weeks later, and therefore, she was discharged on the above treatment regimen. One month later, mycobacterium culture of the lung biopsy tissue was found to be positive for M. intracellulare, which was sensitive to clarithromycin, ethambutol, amikacin, and rifabutin. Therefore, rifabutin was substituted for rifampicin in the treatment regimen. The patient was continuing in remission and her imaging results showed an improvement. At the time of writing this report the patient had been on treatment for 15 months and will remain on the current treatment regimen with regular follow-up.
DISCUSSION
NTM is the general name of Mycobacterium (M.), except M. tuberculosis and M. leprae, which widely exists in soil, water and dust in the environment.1 To date, more than 180 species of NTM have been identified, with MAC being the most common pathogen in NTM infections.2 NTM infections are classified into 4 clinical types: pulmonary disease, lymphadenitis, cutaneous soft tissue disease and disseminated disease.3 Disseminated NTM infections, typically found in immunocompromised individuals, are rare, with an estimated incidence of 1.0 to 1.8 per 100 000 persons.4 In the case of the patient described above, as she had no previous medical history other than hypertension, the differential diagnosis was initially focused on malignant solid or haematological tumours. Therefore, the possibility of opportunistic infections was ignored until the sternal biopsy suggested granulomatous inflammation.
Interferon-γ (IFN-γ), a key cytokine produced mainly by activated CD4+ T lymphocytes and NK cells, plays a crucial role in the cell-mediated immune response to intracellular pathogens. Our patient’s total T lymphocyte, CD4+ T lymphocyte and NK cell counts were all lower than normal, resulting in reduced IFN-γ production and decreased cellular immune function, thus leading to the spread of infection. Interestingly, neutralizing anti IFN-γ antibodies have been detected in many previously healthy Asian adults.5 These antibodies bind to the functional epitopes of IFN-γ, inhibit IFN-γ activity and exacerbate mycobacterial disease.6
Acid-fast bacilli smear microscopy and culture are the gold standard for the diagnosis of mycobacteria. However, smear microscopy has low sensitivity and cannot distinguish M. tuberculosis from NTM,7 while culture is time-consuming. Conventional molecular biological techniques, such as polymerase chain reaction (PCR), line probe hybridization, and DNA sequencing, have limited sensitivity and specificity, and sometimes even misidentify the subspecies of mycobacteria.8 mNGS, an emerging molecular biology diagnostic technology, has overcome these disadvantages. It can determine the genomic information of all DNA and RNA in the sample to identify and type all pathogenic microorganisms, with the advantages of high throughput, wide coverage, fast speed and high sensitivity, potentially revolutionizing the surveillance and diagnosis of infectious diseases. mNGS was invaluable in our patient, resulting in rapid and accurate diagnosis and prompt implementation of the recommended anti-MAC regimen. Amongst other uses, mNGS can also identify gene mutations encoding drug resistance and track nosocomial infections.9
In conclusion, disseminated NTM infection should be considered when multi-systemic lesions involving lymph nodes, lungs and bones are encountered. Moreover, in such cases, mNGS can be used to make early and accurate diagnosis and guide treatment. We suggest that NTM should be ruled out by mNGS in patients with granulomatous lesions that cannot be diagnosed by routine examination. Anti IFN-γ autoantibody testing should be scheduled if a previously healthy patient, especially one from Southeast Asia, develops disseminated NTM disease.
References
- Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56:2000535.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic characterization of nontuberculous mycobacteria. Sci Rep. 2017;7:45258.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of nontuberculous mycobacterial disease in children. J Paediatr Child Health. 2021;57:15-18.
- [CrossRef] [PubMed] [Google Scholar]
- An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-16.
- [CrossRef] [PubMed] [Google Scholar]
- Natural history and evolution of anti-interferon-?? autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis. 2020;71:53-62.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in the diagnosis of tuberculosis-Journey from smear microscopy to whole genome sequencing. Indian J Tuberc. 2020;67(Suppl):S61-S68.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with acquired anti IFN-? autoantibody in patients with nontuberculous mycobacterial infection. PLoS One. 2017;12:e0176342.
- [CrossRef] [PubMed] [Google Scholar]
- Nontuberculous mycobacteria in respiratory infections: Advances in diagnosis and identification. Clin Lab Med. 2014;34:271-95.
- [CrossRef] [PubMed] [Google Scholar]
- Whole genome sequencing in the management of non-tuberculous mycobacterial infections. Microorganisms. 2021;9:2237.
- [CrossRef] [PubMed] [Google Scholar]




