Translate this page into:
Retinopathy secondary to flare-up of systemic lupus erythematosus
[To cite: Kumar J, Chandrappa D, Sen S, Sivakumar R. Retinopathy secondary to flare-up of systemic lupus erythematosus. Natl Med J India 2023;36:26–8. DOI: 10.25259/NMJI_470_20]
Abstract
Systemic lupus erythematosus (SLE) can have widespread ocular manifestations, and posterior segment involvement may be associated with poor visual outcome. We report a clinical flare-up of SLE presenting as combined vascular occlusion in one eye and drusen-like deposits, which is a newly described entity in both eyes. As an ophthalmologist, a knowledge of such presentations helps us identify and possibly help the rheumatologist titrate treatment accordingly, to prevent severe life-threatening systemic complications.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex autoimmune multi-systemic connective-tissue disorder. Acute flare-ups of SLE can lead to catastrophic sequelae, which may include life-threatening complications.1 Many ocular complications occur in SLE, and posterior segment involvement in the form of vasculitis or thrombotic manifestations may result in major loss of vision. We report a patient of SLE, who developed combined vascular occlusion in one eye following dengue fever and gastrointestinal complications. The ophthalmic features were an indicator of the severity and a marker of SLE flare-up.
THE CASE
A 43-year-old woman diagnosed with SLE was referred to us from a tertiary hospital with complaints of diminution of vision in her left eye (OS) for 2 days and right eye (OD) for 1 month. She was on immunosuppressive therapy for the past 3 years. Two months back, the patient had an acute febrile illness, which was diagnosed as dengue fever, and during this treatment, she reported that her immunosuppressive medication had become irregular. One month later, after recovery from dengue, she developed sudden onset abdominal pain, which was diagnosed as ileal gangrene, for which she had to undergo laparotomy and ileostomy. She also developed dry gangrene of the digits, which was diagnosed as a complication of SLE vasculitis flare-up (Fig. 1).

- Clinical photograph of the left hand showing dry gangrene of fingers
On presentation to us, the best-corrected visual acuity was no perception of light OD and 6/60 OS. Anterior segment examination was within the normal limits in both eyes (OU). Pupillary examination revealed a relative afferent pupillary defect OD. Dilated fundus examination OD revealed a pale disc, sclerosed vessels (arteries and veins) in all quadrants with extensive superficial retinal haemorrhages, along with the presence of extensive drusen-like deposits (DLDs) in the posterior pole. Fundus OS also revealed extensive DLDs, with mild temporal optic nerve head pallor.
Fundus fluorescein angiography (FFA) showed a complete lack of filling in arteries as well as veins till the late-phase, confirming a diagnosis of combined vascular occlusion. Staining of the DLDs was also noted in the FFA OD (Fig. 2a). Optical coherence tomography (OCT) revealed extensive DLDs and the presence of hyper-reflective and disorganized inner retinal layers OD (Fig. 2b). FFA OS also showed staining of DLDs throughout the mid- and late-phases, and their presence was confirmed on OCT (Figs 3a, b).
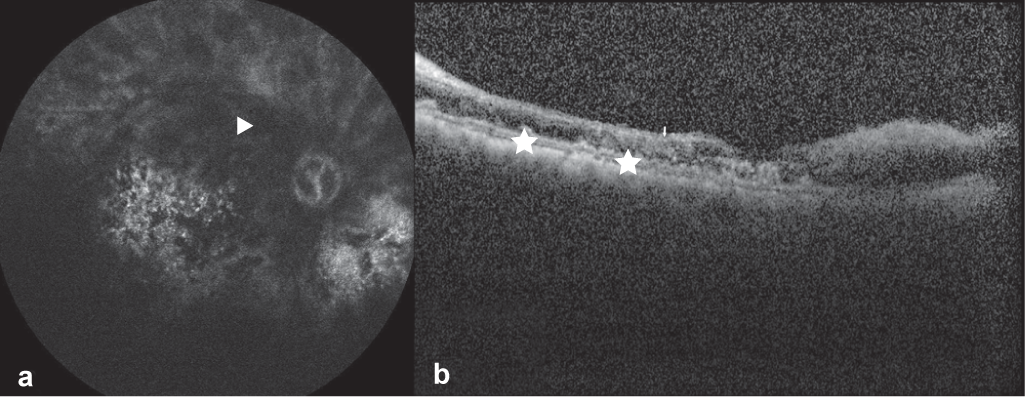
- Late phase fundus fluorescein angiography (Heidelberg Spectralis) image of the right eye showing complete lack of filling of the arteries and veins diagnostic of combined vascular occlusion (arrowhead) along with staining of drusen-like deposits (a) and Spectral-Domain Optical Coherence Tomography (Heidelberg Engineering, Inc., Heidelberg, Germany) image using high definition raster scan of the right eye showing thinning and disorganization of the inner retinal layers and sub-retinal pigment epithelium deposition of drusen-like deposits (asterix) (b)
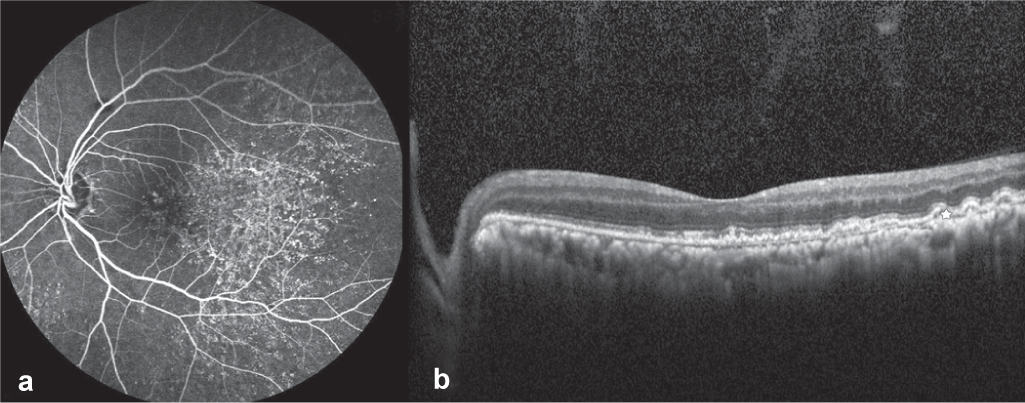
- Late phase fundus fluorescein angiography (Heidelberg Spectralis) image of the left eye showing normal filling of the arteries and veins and staining of drusen-like deposits throughout the mid and late phase (a) and Spectral-Domain Optical Coherence Tomography (Heidelberg Engineering, Inc., Heidelberg, Germany) images using high definition raster scan of the left eye showing sub-retinal pigment epithelium deposition of drusen-like deposits (b)
The patient had undergone serological tests for SLE, which were strongly positive for lupus anticoagulant and antinuclear antibody (IgG). Complete haemogram revealed microcytic hypochromic anaemia, with mild leucocytosis and kidney function tests revealed a serum creatinine of 2.3 mg/dl. Magnetic resonance imaging of the brain and orbit was suggestive of a non-compressive optic neuropathy OS. A final diagnosis of OU DLDs with OD combined arteriovenous occlusion secondary to the acute flare-up of SLE was made.
After 8 months of follow-up, OD visual acuity did not change from no perception of light, and the patient developed neovascularization of the iris, for which a scatter pan-retinal photocoagulation OD was done. OS visual acuity was stable at 6/60. At 11 months follow-up, OU optic disc showed pallor. OCT image OD showed atrophic retinal layers with existing DLD retinal pigment epithelium undulations.
DISCUSSION
SLE is a chronic multi-system autoimmune disease characterized by the production and deposition of immune complexes in tissues throughout the body. Ocular involvement in SLE was first described by Semon and Wolff and is said to be a marker of overall systemic disease activity, seen in almost 30% of all patients.2 Keratoconjunctivitis sicca or dry eye is the most common ocular manifestation.3 Retinal involvement/SLE retinopathy may occur in 7%–26% and present as mild immune-mediated microangiopathy, similar to diabetic and hypertensive retinopathy, including cotton wool spots, microaneurysms, hard exudates or dot-blot haemorrhages.2 In severe stages, there can be multiple vessel occlusion involving arteries or veins, presenting with signs of ischaemia. Both the mild and severe stages of SLE retinopathy may be associated with central nervous system (CNS) disease and highly active SLE pathology.4,5 Retinopathy may also present uncommonly as vasculitis appearing as vascular sheathing. Renal involvement in SLE may also lead to secondary hypertension, which may be associated with the progression or appearance of new changes of hypertensive retinopathy.
Vision-threatening diseases of retina can occur at any time in the course of SLE, either as an initial manifestation or as a flare-up of the underlying systemic disease. While milder SLE retinopathy may be the result of immune-complex deposition and inflammation, more severe vaso-occlusion may arise from fibrinoid degeneration/necrosis without much inflammation.
There have been case reports in the past on combined vascular occlusion in SLE, and a few of them have shown its association with clinical flare-up of the disease.6,7 However, none of them have shown any simultaneous or associated serious systemic complications such as ileal gangrene or gangrene in peripheral extremities, as was in this patient. Previous longitudinal studies have shown that patients with SLE retinopathy may have reduced survival, hence detecting the retinal lesions promptly may be important both for visual and systemic prognosis.8–11
Our patient had a high level of lupus anticoagulant. The antiphospholipid antibody or lupus anticoagulant is prothrombotic and has been reported to be associated with CNS and ocular vaso-occlusive phenomena in patients with SLE.12,13
DLDs in SLE are a newly described entity and the literature describing these is scanty. The presence of these DLDs is said to be associated with renal involvement in SLE patients. DLDs are possibly immune-complex deposits at the level of choroidal stroma and Bruch’s membrane.14 Renal involvement in SLE can occur even in the absence of any obvious clinical manifestations with normal renal parameters, termed as silent lupus nephritis. Moreover, renal dysfunction may be present without retinopathy in 60% of patients. However, in retinopathy patients, renal dysfunction has been reported in 100% of cases.13 Although there is still no consensus regarding the association between SLE retinopathy and renal disease, since SLE retinopathy generally develops in the more severe stages of SLE, a kidney involvement should be suspected and investigated for, as seen in our case. The presence of these DLDs could be an indicator of such a systemic disease. Invernizzi et al. have pointed out that these DLDs occurring in patients with obvious renal involvement are numerous in distribution as compared to the ones with possible silent lupus nephritis.15 Hence, the occurrence of DLDs should promptly necessitate an evaluation of a SLE patient for possible lupus nephritis, although more studies are needed to establish this association. DLDs have been very well documented in our case, and the patient also had an associated renal impairment.
Optic nerve involvement in patients with SLE may be in the form of optic neuritis, ischaemic optic neuropathy and papillodema. Visual outcome following optic neuropathy is generally optimum, with good outcomes reported in a few cases.16
Treatment of SLE retinopathy should be in the lines of controlling the systemic disease by systemic corticosteroids and immunosuppressives.16 Our case report is unique as it depicts the sequential appearance of systemic and ocular complications after a presumed flare-up of SLE due to dengue fever as well as irregular immunosuppressive therapy. It also shows classical OCT and FFA images of bilateral DLDs in SLE. We hope it will help practitioners, especially ophthalmologists, to become aware of and foresee the possible life-threatening and vision-threatening complications of SLE. Ophthalmologists can play a crucial role in helping the rheumatologist modify the treatment regimen of SLE to prevent further life-threatening systemic complications.
Conflicts of interest
None declared
References
- Presentation of systemic lupus erythematosus (SLE) in emergency department: A case report. BMC Res Notes. 2013;6:181.
- [CrossRef] [PubMed] [Google Scholar]
- Acute lupus erythematosus, with fundus lesions. Proc R Soc Med. 1933;27:153-7.
- [CrossRef] [Google Scholar]
- Diagnosis and treatment of uveitis (2nd ed). Jaypee Brothers Medical Publishers: New Delhi; 2013.
- [Google Scholar]
- Severe retinal vaso-occlusive disease in systemic lupus erythematous. Arch Ophthalmol. 1986;104:558-63.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular manifestations of systemic lupus erythematosus. Curr Opin Ophthalmol. 2008;19:512-18.
- [CrossRef] [PubMed] [Google Scholar]
- Combined central retinal artery and vein occlusion in lupus. BMJ Case Rep. 2017;2017:Bcr-201621884.
- [CrossRef] [PubMed] [Google Scholar]
- Acute vision loss in systemic lupus erythematosus: Bilateral combined retinal artery and vein occlusion as a catastrophic form of clinical flare. Lupus. 2018;27:1023-6.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic lupus erythematosus choroidopathy. Available at www.uveitis.org/wp-content/uploads/2017/05/systemic_lupus_erythematosus_choroidopathy.pdf (accessed on 30 Mar 2020)
- [Google Scholar]
- Principles and practice of ophthalmology. Philadelphia: WB Saunders; 1994. p. :2894-1.
- [Google Scholar]
- Ocular findings in systemic lupus erythematosus. Br J Ophthalmol. 1972;56:800.
- [CrossRef] [PubMed] [Google Scholar]
- Retinopathy in systemic lupus erythematosus: Relationship to disease activity. Arthritis Rheum. 1986;29:1152-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association of antiphospholipid antibodies with retinalvascular disease in systemic lupus erythematosus. Semin Arthritis Rheum. 1999;28:326-2.
- [CrossRef] [PubMed] [Google Scholar]
- Role of lupus retinopathy in systemic lupus erythematosus. J Ophthalmic Inflamm Infect. 2016;6:15.
- [CrossRef] [PubMed] [Google Scholar]
- Choroidopathy in patients with systemic lupus erythematosus with or without nephropathy. J Nephrol. 2011;24:522-9.
- [CrossRef] [PubMed] [Google Scholar]
- Drusen-like deposits in young adults diagnosed with systemic lupus erythematosus. Am J Ophthalmol. 2017;175:68-76.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100:135-41.
- [CrossRef] [PubMed] [Google Scholar]




