Translate this page into:
Tachycardiomyopathy managed by successful ablation of right ventricular outflow tract premature ventricular complexes
Correspondence to KRISHNA KUMAR MOHANAN NAIR; kakkam@gmail.com
To cite: Raghuram K, Mohanan Nair KK, Namboodiri N, Valaparambil A. Tachycardiomyopathy managed by successful ablation of right ventricular outflow tract premature ventricular complexes. Natl Med J India 2021;34:211–13.
Abstract
Tachycardiomyopathy is a common reversible cause of left ventricular dysfunction. Prompt diagnosis and treatment of this condition is essential to ensure a good prognosis for the patient. We report a case of tachycardiomyopathy due to frequent premature ventricular complexes arising from the right ventricular outflow tract midseptum managed with successful ablation.
INTRODUCTION
Tachycardiomyopathy, also termed as arrhythmia-induced cardiomyopathy, is defined as the reversible impairment of ventricular function induced by persistent arrhythmia.1 Delay in diagnosis and management of this condition can result in considerable morbidity. Management of tachycardiomyopathy involves prompt treatment of the underlying arrhythmia.
THE CASE
A 48-year-old woman presented with complaints of palpitations and fatigue for the past 5 years. She had initially sought treatment at a nearby hospital where she was evaluated for coronary artery disease. A coronary angiogram done in 2015 revealed normal coronaries. She was kept on medical follow-up but her symptoms gradually worsened. In January 2019, the patient again sought medical care for palpitations and fatigue. Holter test revealed a premature ventricular complex (PVC) burden of 47% and repetitive runs of non-sustained ventricular tachycardia (NSVT). She was started on sustained-release metoprolol, but her symptoms did not improve. She was then referred to us for further management.
At presentation, the patient complained of functional class III palpitations and fatigue; vitals were stable. An electrocardiogram (ECG) done at that time revealed sinus rhythm with multiple PVCs, heart rate of 80 beats per minute, sinus beat axis of 60°, PR interval of 160 ms, QRS duration of 80 ms, PVC axis was inferior with early transition in lead V3, with intermittent runs of NSVT arising from the anterior right ventricular outflow tract (RVOT) septum. Chest X-ray showed no cardiomegaly with grade 1 pulmonary vascular hypertension. Thyroid function was normal. An echocardiogram (ECHO) showed moderate left ventricular dysfunction with an ejection fraction of 37% and global left ventricular hypokinesia. N-terminal-pro B-type natriuretic peptide (NT-pro BNP) at presentation was found to be 563 pg/ml.
An electrophysiological study with radiofrequency ablation under three-dimensional (3D) EnSite mapping (EPS+RFA under 3D) was done. Using a femoral venous access, a quadripolar catheter and a Livewire large curve ablation catheter were used. The baseline study showed similar PVCs and runs of NSVT, as noted in the ECG (Fig. 1). Activation mapping was done and the point of earliest activation was noted in the RVOT midseptum (earlier than the surface ECG by 53 ms) (Fig. 2). Ablation at the mid-RVOT septum and consolidation ablation was done (power max 50 W, temperature 55 °C, time 180 seconds; Fig. 3). Post-procedure ECG showed no PVCs, and the patient confirmed that her palpitations had resolved.

- Baseline electrocardiogram at the start of electrophysiological study showing multiple premature ventricular complexes arising from right ventricular outflow tract septum with intermittent runs of non-sustained ventricular tachycardia
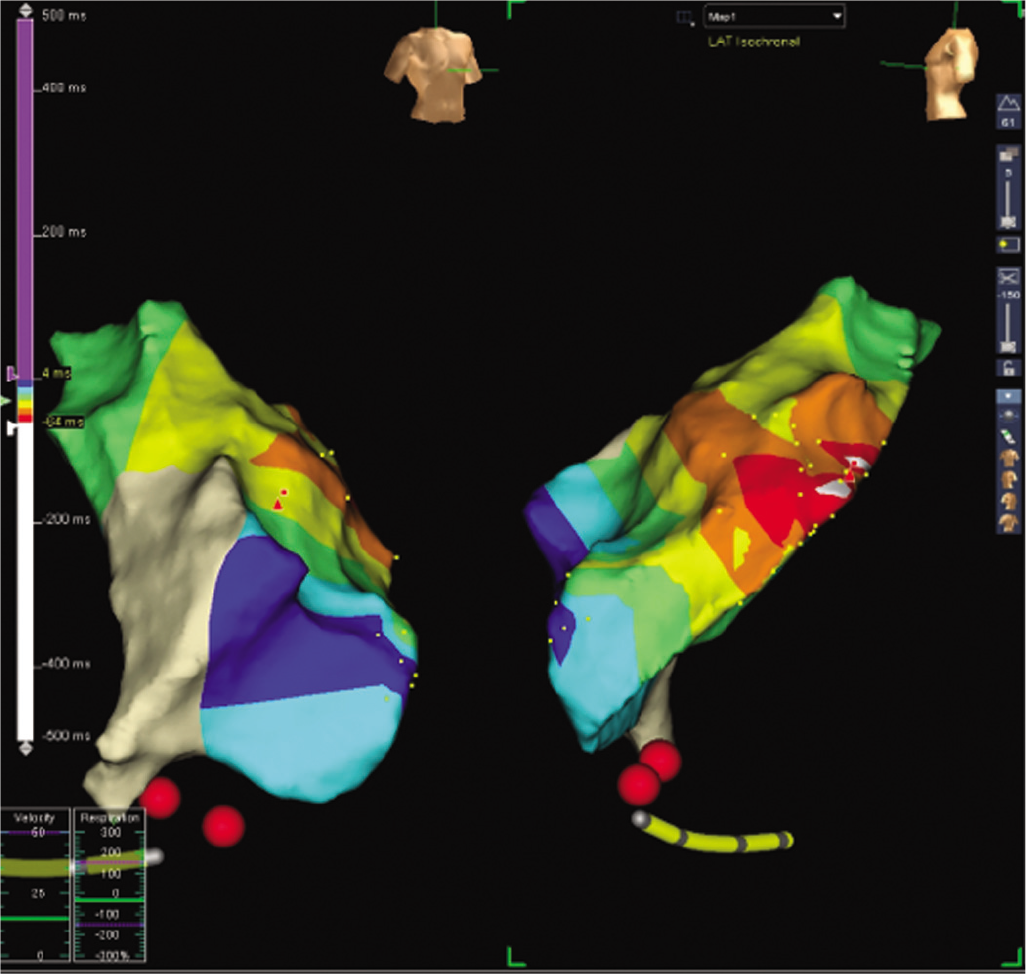
- Activation mapping using three-dimensional EnSite system showing right ventricular outflow tract midseptum is earlier than surface electrocardiogram by 53 ms
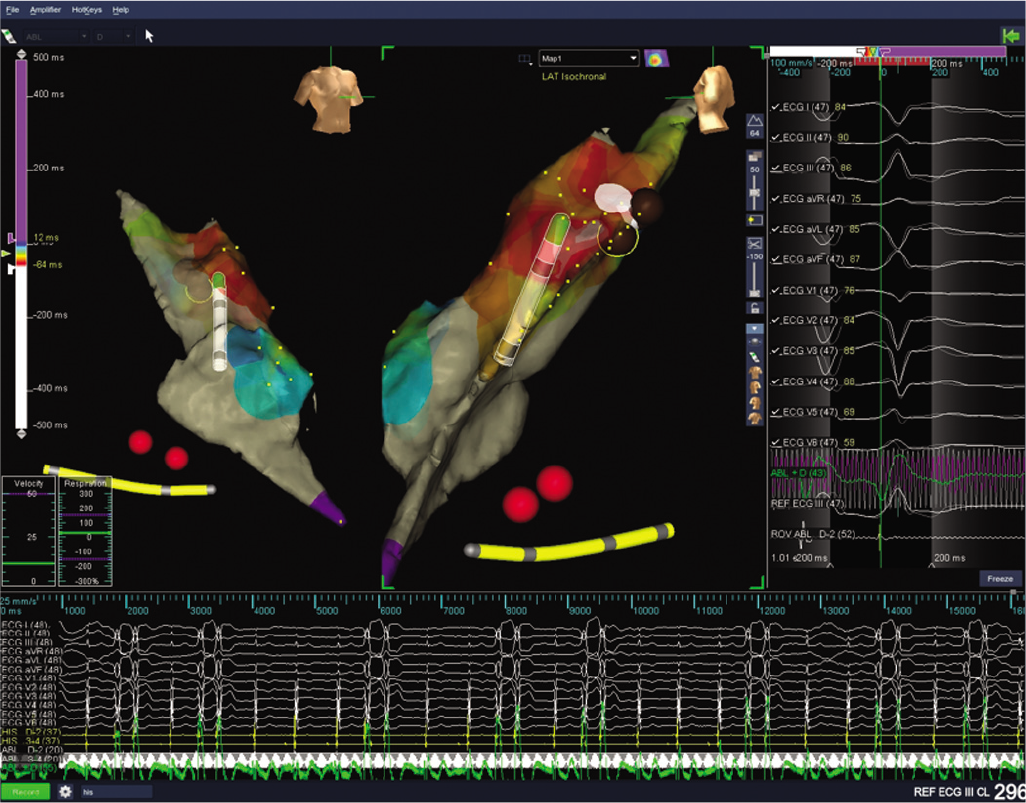
- Ablation burn delivered to right ventricular outflow tract midseptum
On follow-up assessment after 1 month, the patient was symptomatically better with functional class I. ECG was normal with no PVCs. ECHO showed a good left ventricular function with ejection fraction 61%. NT-pro BNP levels had reduced to 198 pg/ml. Holter test was repeated on follow-up, which showed a PVC load of 0%. The patient continues to be asymptomatic on follow-up not requiring medical therapy.
DISCUSSION
Tachycardiomyopathy is an important cause of cardiac dysfunction. It is defined as ‘atrial and/or ventricular dysfunction secondary to rapid and/or synchronous/irregular myocardial contraction, partially or completely reversed after the treatment of the causative arrhythmia’.2 Other causes of reversible cardiomyopathy need to be excluded. Inflammatory cardiomyopathy occurs due to inflammatory disease of the myocardium with associated cardiac dysfunction. There are multiple infectious and non-infectious causes for inflammatory cardiomyopathy. A detailed history with a history of exposure to toxins, cytotoxic chemotherapy and evidence of infection with coxsackievirus, cytomegalovirus, parvovirus and mumps virus is attributed to the development of inflammatory cardiomyopathy. Conservative treatment for heart failure is the first line of management as most cases of inflammatory cardiomyopathy resolve over time.3
Peripartum cardiomyopathy (PPCM) should be considered in patients developing heart failure either during the last month of pregnancy or within the first 5 months after delivery. Management of PPCM includes treatment of heart failure. About 30%–50% of patients have considerable improvement in cardiac function in the first 6 months after the onset of heart failure.3
Thyroid disease-induced cardiomyopathy is a cause of reversible cardiomyopathy due to an imbalance of thyroid hormones. Management includes correction of the underlying thyroid disorder along with managing the heart failure. Another important point to note in patients of hyperthyroidism is the development of atrial fibrillation, which in turn is associated with tachycardiomyopathy.3
Based on the presence or absence of underlying heart disease, tachycardiomyopathies can either be arrhythmia-induced, where the arrhythmia is the only reason for cardiac dysfunction, or arrhythmia-mediated, where the arrhythmia worsens cardiac function in a patient with structural heart disease.4 It is important to differentiate PVCs occurring due to underlying cardiomyopathy such as arrhythmogenic right ventricular dysplasia (ARVD) from those that arise from the RVOT as there are widespread differences in prognosis and management. Similarities include ventricular arrhythmias with left bundle branch morphology, inferior axis and precipitation of symptoms in young otherwise healthy individuals. Emkanjoo et al. studied the electrocardiographic differences in sinus rhythm between patients with ARVD and those with RVOT VT and noted that the mean QRS duration, S wave upstroke duration, JT interval dispersion and JT interval duration in the right and left precordial leads were more in patients with ARVD than in those with RVOT VT. T inversion in V1–V3, though found more commonly in patients with ARVD, had a low sensitivity and specificity in differentiating patients with ARVD from those with RVOT VT.5
The pathophysiological mechanisms of tachycardiomyopathy are multiple, and proposed mechanisms include variations in cardiomyocyte energy metabolism, subclinical ischaemia, calcium overload and redox stress.6 All these mechanisms can be reversed by treating the underlying tachycardia. Thus, the early recognition of tachycardiomyopathy and its prompt treatment is of vital importance to the overall management of this condition.
An important cause of tachycardiomyopathy is idiopathic VT. Most commonly arising from the RVOT, Hasdemir et al. showed that 11% of patients presenting with frequent PVCs had sustained monomorphic VT and 7% of these patients had tachycardiomyopathy.7 A PVC burden ranging from >10 000 to 25 000 PVCs per day and as >10% to 24% of total heartbeats/day is considered high, and a threshold burden of 10 000 PVCs per day is considered to be associated with development of tachycardiomyopathy.8
Penela et al. noted that a threshold of 13% PVC burden per day was associated with left ventricular dysfunction.9 They also reported that ablation of >13% baseline PVC burden had a 100% sensitivity and 85% specificity to predict an absolute increase in left ventricular ejection fraction. Mountantonakis et al. reported that ventricular function can improve if PVC burden is reduced to <5000 per day.10
Conclusion
This case exemplifies the management of tachycardiomyopathy with ablation of the underlying arrhythmia. The improvement in functional class, increase in ejection fraction and reduction in NT-pro BNP levels objectively confirm this.
Conflicts of interest
None declared
References
- Pathophysiology, diagnosis and treatment of tachycardiomyopathy. Heart. 2017;103:1543-52.
- [CrossRef] [PubMed] [Google Scholar]
- Arrhythmia-induced cardiomyopathies: The riddle of the chicken and the egg still unanswered? Europace. 2012;14:466-73.
- [CrossRef] [PubMed] [Google Scholar]
- Reversible cardiomyopathies. Clin Med Insights Cardiol. 2015;9:7-14.
- [CrossRef] [PubMed] [Google Scholar]
- Arrhythmia-induced cardiomyopathies: Mechanisms, recognition, and management. J Am Coll Cardiol. 2015;66:1714-28.
- [CrossRef] [PubMed] [Google Scholar]
- Electrocardiographic (ECG) clues to differentiate idiopathic right ventricular outflow tract tachycardia (RVOTT) from arrhythmogenic right ventricular cardiomyopathy (ARVC) Indian Heart J. 2014;66:607-11.
- [CrossRef] [PubMed] [Google Scholar]
- Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709-15.
- [CrossRef] [Google Scholar]
- Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: The incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663-8.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: A prospective multicenter study. J Am Coll Cardiol. 2013;62:1195-202.
- [CrossRef] [PubMed] [Google Scholar]
- Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: Effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608-14.
- [CrossRef] [PubMed] [Google Scholar]




