Translate this page into:
The Origin and Evolution of Critical Laboratory Values
[To cite: Lundberg GD. The origin and evolution of critical laboratory values. Natl Med J India 2023;36:283–5. DOI: 10.25259/NMJI_1051_2023]
‘One evening in 1969, an unaccompanied young man was admitted to the Los Angeles County-University of Southern California Medical Center in coma of unknown etiology, after having been found unconscious in a downtown hallway. When a physical examination disclosed a laceration of the scalp, he was admitted to the neurosurgical service. Shortly thereafter CBC (complete blood count), urinalysis, and serum electrolytes were ordered and the proper specimens were sent to the laboratory. Deep coma persisted. All laboratory results were normal except for a serum glucose of 6 mg%. The hard-copy laboratory results were returned to the ward of origin within two hours of receipt of the specimens in the laboratory. However, the results were not noticed by the house officers who were busy with several other seriously ill patients. Ward personnel also failed to communicate the lab results to the responsible physicians. The following morning, when an intern noticed the laboratory value, he administered glucose immediately, but there was no response. Irreversible brain damage had occurred; the patient died soon thereafter.’1
A select few laboratory results represent a pathophysiological state at such variance with normal as to be life-threatening, unless something is done quickly, and for which a life-saving intervention can be done quickly.
Our response to this episode initiated the ‘critical value recognition and reporting system’.
Our medical centre was a large public hospital with many very sick patients, so we called the new numbers ‘Panic values’. Critics complained that good doctors should never panic so the name was changed to ‘Critical values’. The original list included only: serum sodium, potassium, glucose, calcium and bicarbonate, prothrombin activity, arterial or capillary blood PO2 and PCO2, platelet count, packed red blood cell (RBC) volume, blood haemoglobin, positive blood culture and positive cerebrospinal fluid (CSF) Gram-stain.
At that time we required the responsible laboratory person to quickly verify the result and use the telephone (long before laboratory computers) to personally notify a responsible individual (no messages left) who agreed to find a physician who could quickly act on the result. All was documented with times and names.
The list was brief; the urgency obvious; the actions understood without question. The system worked. I was invited by a visiting editor to write it up and the non-peer-reviewed controlled circulation magazine Medical Laboratory Observer published it with a multicoloured chart intended for bulletin-board posting (Fig. 1).
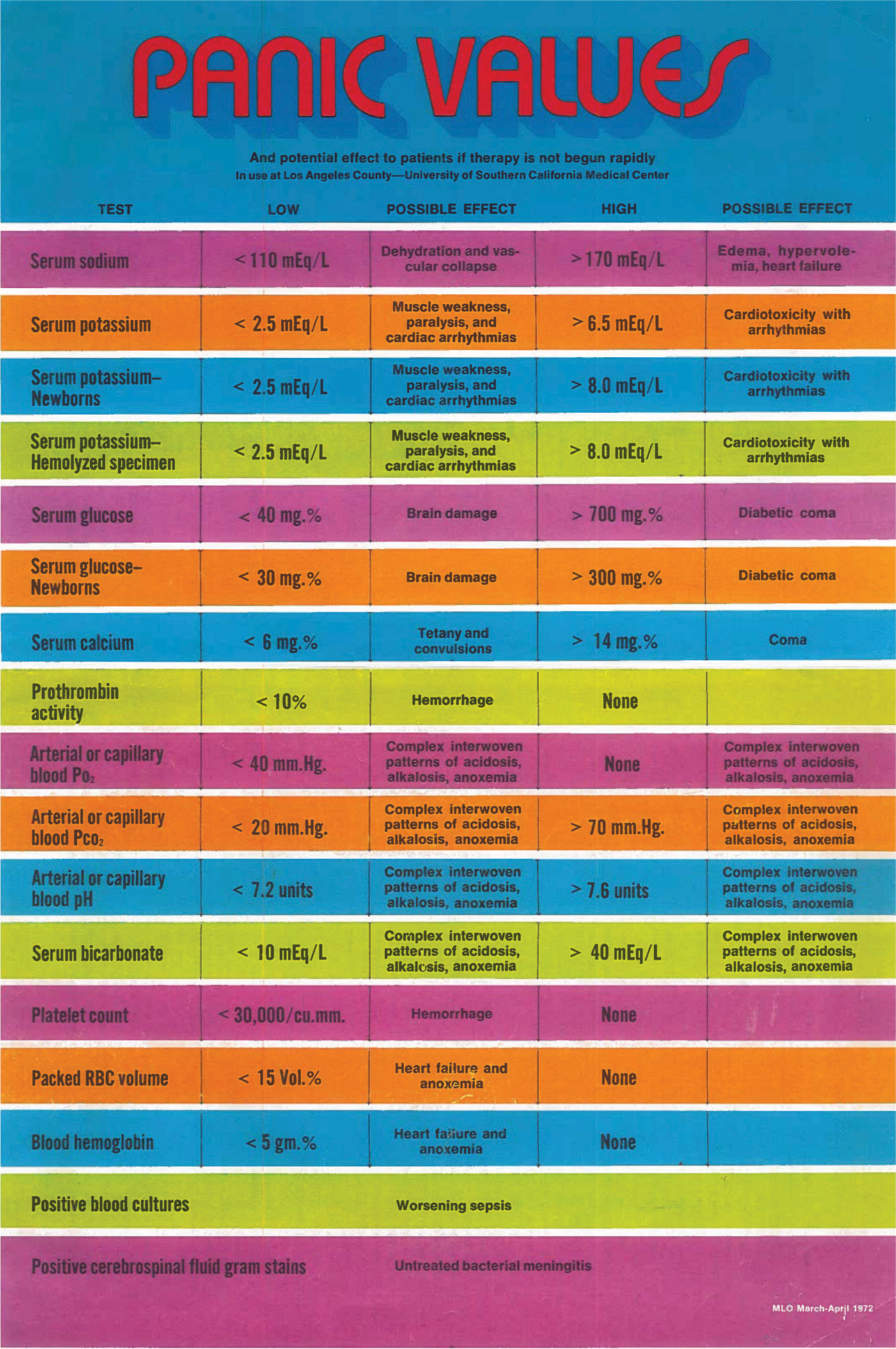
- The multi-coloured chart as it was published in the Medical Laboratory Observer in 1972
Within weeks, laboratories all over the USA adopted their own version of the system. The tests chosen and critical values were established by each medical staff. Speaking bureaucratically, a critical value system ‘quickly’ became standard of practice as required by the College of American Pathologists Laboratory Accreditation Program and the Joint Commission on Accreditation of Hospitals.
We later expanded the programme to include ‘Vital values’, which represented laboratory findings every bit as important for action as ‘Critical values’ but for which timing was less urgent. Examples were a positive Pap smear for cancer of the cervix, a positive culture of sputum for tuberculosis, or a positive mammogram for cancer.2
Most laboratory tests that are done do not need to be done; the results are either negative, normal or show no change from a prior result. But some are crucial.
The critical value system rapidly became a standard of practice and its use remains ubiquitous. Remarkably little change has occurred in the intervening 50 years, although additional tests and values have been added, mostly of the ‘Vital’, not ‘Critical’ categories. We always said that each institution medical and pathology staff should pattern its own. The main changes have come from at what level a given institution’s staff might push the ‘Panic button’.
Before publishing these observations in 1972, but as a part of our rethinking of what clinical laboratory testing was all about, we challenged the concept of what a laboratory test consisted of.3 Most laboratory people considered the study of a specimen to produce a result to be a ‘test’. We changed that to define a laboratory test as consisting of nine steps, which we termed the ‘Brain to Brain Loop’. Need recognized and test ordered; specimen collected; identification; transportation; processing; analysis; reporting; interpretation; action taken. We stated that anything that interferes with the process at any stage represents a failed test. Later we added a tenth step: outcome attributed to the laboratory test.4
Recognition of this event and related re-thinking began a series of changes in laboratory organization and function which cascaded into a worldwide recognition of the importance of patient-centredness.
Starting with the Laboratory utilization committee, we applied the patient-focused approach to laboratory management across all fields.
We established patient-focused committees consisting of clinicians and laboratorians for chemistry, toxicology, haematology and microbiology.
When a person gets sick, they get sick. It does not matter what day of the week or time of day it is. And, a clinical laboratory should be agnostic in its ability to respond, 24x7.
We reorganized haematology, chemistry and toxicology strictly according to turnaround time (TAT) of tests. We ‘started the clock’ any and all days/times 24×7 when a specimen arrived at some place within the laboratory and stopped the clock when a final result was available somewhere in the laboratory. We categorized all tests as: less than 1 hour, less than 4 hours, less than 24 hours, and more than 24 hours, guaranteed, 24×7. As a trade-off, we abolished the concept of ‘stat’ orders…NO EXCEPTIONS. The rationale of each TAT was the speed with which a result was needed to render proper medical care that mattered to the welfare of the patient, and, of course, that was technically possible.
We understand that when a physician wants something, he/she wants it, no matter what. Well, in this patient-focused approach, the physician cannot have it, except as offered by the patient-focused approach, based on TAT.
We described this radical approach to laboratory organization in a full book titled Managing the patient-focused laboratory.5
I am gratified that articles by various writers about the critical value system have appeared every decade since its original description 50 years ago. The original concept, and even the exact wording used to describe the basics, have survived intact.6–8
Conflicts of interest
None declared
References
- It is time to extend the laboratory critical (panic) value system to include vital values. Med Gen Med. 2007;9:20.
- [Google Scholar]
- Acting on significant laboratory results. JAMA. 1981;245:1762-3.
- [CrossRef] [PubMed] [Google Scholar]
- Adding outcome as the 10th step in the brain-to-brain laboratory test loop. Am J Clin Pathol. 2014;141:767-9.
- [CrossRef] [PubMed] [Google Scholar]
- Managing the patient-focused laboratory Oradell, NJ: Medical Economics Co.; 1975. p. :379.
- [Google Scholar]
- Critical limits for urgent clinician notification at US medical centers. JAMA. 1990;263:704-7.
- [CrossRef] [PubMed] [Google Scholar]
- Communication of critical laboratory values: Optimization of the process through secure messaging. Lab Med. 2020;51:e6-e11.
- [CrossRef] [PubMed] [Google Scholar]
- A subpage of the directory. Available at https://stanfordlab.com/test-directory/critical-values.html (accessed on 20 Sep 2023)
- [Google Scholar]




