Translate this page into:
Thyroid hormone resistance due to a novel mutation in thyroid hormone receptor presenting as attention deficit hyperactivity disorder
Correspondence to BINDU KULSHRESHTHA; drbindu25@yahoo.co.uk
[To cite: Yadav R, Goyal M, Aggarwal A, Kulshreshtha B. Thyroid hormone resistance due to a novel mutation in thyroid hormone receptor presenting as attention deficit hyperactivity disorder. Natl Med J India 2024; 332–4. DOI: 10.25259/NMJI_869_2022.]
Abstract
Resistance to thyroid hormone (RTH) is rare and is characterized by high circulating levels of thyroid hormones in the presence of either normal or elevated thyroid stimulating hormone (TSH) levels. Decreased responsiveness of the peripheral tissues to thyroid hormones owing to defective thyroid receptor function is the underlying cause. RTH is variable in its presentation. We report a 21-year-old man with long-standing attention deficit hyperactivity disorder (ADHD) and learning disabilities. His thyroid function tests showed an increased free T3 and T4 in the presence of a non-suppressed TSH. Other pituitary hormones were normal. Subsequently, a genetic analysis revealed a heterozygous mutation (Pro452Thr) in THRβ gene, establishing the diagnosis of RTH. Thus, this was a RTH presenting as ADHD due to a novel mutation in the thyroid hormone receptor gene.
INTRODUCTION
Resistance to thyroid hormone (RTH) is due to mutations in the thyroid hormone receptor (THR) gene. It is characterized by high circulating levels of thyroid hormones in the presence of either normal or elevated levels of thyroid stimulating hormone (TSH), due to decreased responsiveness of the peripheral tissues to thyroid hormones. The syndrome is variable in its presentation as high circulating thyroid hormones compensate for the tissue resistance to a varying degree.1,2 When the compensation is incomplete, hypothyroidism is found in tissues that express predominantly THRβ such as the liver, kidney, and lung. On the other hand, hyperthyroidism can be seen in tissues that express predominantly THRβ such as the heart, brain and bone.1
We report a rare RTH due to a novel mutation in the THR presenting during childhood as attention deficit hyperactivity disorder (ADHD).
THE CASE
A 21-year-old man was referred to our department for work up of his deranged thyroid function tests. He had normal developmental milestones and enrolled in school at the age of 4 years. His parents said he had recurrent ear discharge during childhood due to chronic otitis media. He also had a history of asthma and nocturnal enuresis in his childhood, both of which resolved when he was about 10 years old.
He was an average student scoring around 75% marks till Class 6. However, his parents noticed that he would make simple calculation errors, as he used to get confused between addition and subtraction. There was a gradual decline in his academic performance. Around the age of 12 years, he was assessed by a psychiatrist, who noted learning disability and deficits in reading, writing, arithmetic calculations, spellings, and visual memory. Attention deficits were noted by the psychiatrist during repeat evaluations and he was diagnosed as ADHD. His intelligence quotient (IQ) was 88. He was advised attention enhancement training but the follow-up for counselling sessions was poor.
He had to take supplementary examinations to pass Class 12. Although his parents enrolled him in a regular college, he could not cope up with the syllabus and dropped out after a year.
Around the age of 18 years, he started complaining of palpitations and intolerance to heat. His palpitations were intermittent and unrelated to physical activities. At the age of 19 years, his thyroid functions were assessed, which showed an increased free T3 and T4 levels in the presence of a non-suppressed TSH. All his reports consistently showed the same pattern (Table I).
| Test (normal range) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Thyroid stimulating hormone (0.3-5.5 μIU/ml) | 1.87 | 1.34 | 1.81 | 1.5 | 2.2 |
| Free T4 (0.8–2 ng/dl) | 2.44 | 3.16 | – | 2.8 | – |
| Free T3 (2.3–4.2 pg/ml) | 6.52 | 7.34 | – | 6.09 | – |
| Total T4 (5.01–12.45 μg/dl) | – | – | 16.5 | 15.7 | 18.5 |
| Total T3 (0.6–1.81 ng/dl) | – | – | 1.89 | 1.85 | 2.03 |
| Anti-thyroid peroxidase antibody (<60 U/ml) | <28 | – | – | – | – |
While his initial thyroid tests were ignored, he presented to us at the age of 21 years. His parents and sister had normal thyroid function tests. However, his maternal grandmother and maternal aunt were being treated for hypothyroidism (thyroid function tests were not available).
His height was 165 cm with a BMI of 25.7 kg/m2. He was as tall as his father and well virilised with normal pubertal development. His resting pulse rate was 90 per minute with a blood pressure of 130/80 mmHg. There was no tremulousness or sweating and his systemic examination was also unremarkable. Ultrasound of the thyroid revealed a bulky gland with multiple variable sized colloid cysts, the largest measuring 5.5×3.7 mm.
A differential diagnosis of TSH secreting tumour/RTH was considered in view of his persistently raised thyroid hormones with non-suppressed TSH. Contrast enhanced MRI of the sella revealed a 2.1×1.0×1.5 mm size lesion. His other pituitary hormones were normal (Table II).
| Parameter (normal range) | Value |
|---|---|
| Follicular stimulating hormone (1.5–9.74 mIU/ml) | 3.99 |
| Leuteinizing hormone (1.8–7.8 mIU/ml) | 1.18 |
| Total testosterone (4.5–28.2 nmol/L) | 7.11 |
| Cortisol (123–626 nmol/L) | 315 |
| Prolactin (3–18.6 ng/ml) | 7.18 |
Genetic analysis revealed a mutation in THRβ gene on exon 10. A heterozygous adenine for cytosine substitution at nucleotide position 1354 was found which switched the codon 452 from proline in normal allele to threonine in the mutated allele. Thus, a diagnosis of RTH was made (Fig. 1). The P452T missense variant was predicted to be damaging by both SIFT (Sorting Intolerant From Tolerant) and Polyphen2 (Polymorphism Phenotyping v2) analysis. He was started on propranolol to which his palpitations responded.
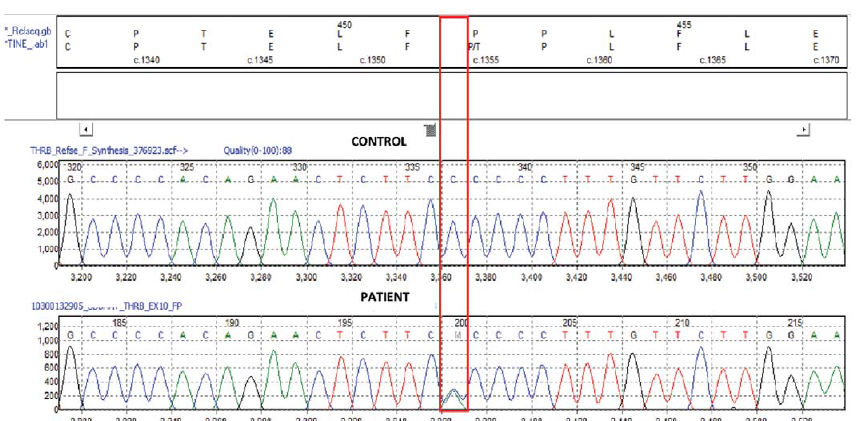
- Genetic analysis
DISCUSSION
RTH is a rare disorder presenting with variable features of hypothyrodism or hyperthyroidism. While around 35% of patients are diagnosed below the age of 10 years, the diagnosis of this disorder is usually delayed until adulthood.3 Our patient was diagnosed at the age of 21 years as he had subtle features of thyroid hormone excess (namely palpitations). Prevalence of tachycardia has been reported in 50%–80% of patients with RTH.2,3 This is attributed to the predominance of alpha receptors in the heart that are stimulated with excess thyroid hormone levels.
While our patient presented at 21 years of age, he had features of learning disabilities and ADHD first noticed during adolescence. The prevalence of ADHD has been reported to be 48%–83% in patients with RTH.4 The pathogenetic basis of the association of ADHD with RTH is largely unknown. Since the brain predominantly expresses TR alpha receptors, excess circulating thyroid hormones provide a thyrotoxic environment to the brain.1 IQ is known to be subnormal in 21%–32% patients, our patient’s IQ was 88.
Our patient had normal growth and pubertal development. Short stature has been reported in only 20% of patients with RTH. Our patient also gave a history of recurrent ear infections in childhood. In a review by Refetoff et al., some patients with THRβ were found to have a higher incidence of recurrent ear, nose and throat infections.1 The reasons for increased susceptibility to these infections is not clear.
Our patient was also found to have a tiny pituitary lesion on MRI. The size of the pituitary lesion was only 2 mm, an incidental finding. Incidental pituitary abnormalities have been reported in patients with RTH. These can range from small pituitary adenomas to pituitary hyperplasia.5–7
The definitive diagnosis of RTH was made by the demonstration of a genetic mutation (Pro452Thr) in the THRβ gene in exon 10. The THRβ gene contains 10 exons, which encode 461 amino acids. Most of the THR mutations interfere with ligand binding, others interfere with TR function through impaired binding to DNA or co-activators. The majority of THRβ variations are found in the ligand-binding domain of the three clustered regions on exons 8, 9 and 10 which encode three hot spots of LBD (codons 309–353, 374–461 and 234–282).8–10 Although there are around 236 mutations reported, this particular mutation (Pro452Thr) has not been reported earlier.9 While functional studies would be conclusive, the P452T missense variant was predicted to be damaging by both SIFT and Polyphen2. Since this variant (Pro452Thr) is located in the ligand-binding domain in the hot spot region, it is possible that the mutation in our proband interferes with the binding of THR to thyroid hormones. The heterozygous nature of the mutation may explain minimal clinical manifestations in our patient.
Thus, our patient had a novel mutation in exon 10 resulting in RTH presenting as ADHD during childhood and the diagnosis was delayed due to a low suspicion of the condition.
References
- The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348-99.
- [CrossRef] [PubMed] [Google Scholar]
- A clinician ¯(’¯s guide to understanding resistance to thyroid hormone due to receptor mutations in the TRá and TRâ isoforms. Clin Diabetes Endocrinol. 2017;3:8.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up of newborns with elevated screening T4 concentrations. J Pediatr. 2003;143:296-301.
- [CrossRef] [PubMed] [Google Scholar]
- Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328:997-1001.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary hyperplasia mimicking thyrotropin-producing pituitary adenoma in the patient with resistance to thyroid hormone: A case report. Int J Neurosci. 2022;132:207-11.
- [CrossRef] [PubMed] [Google Scholar]
- A novel thyroid hormone receptor beta gene mutation (G251V) in a Thai patient with resistance to thyroid hormone coexisting with pituitary incidentaloma. Thyroid/. 2016;26:1804-6.
- [CrossRef] [PubMed] [Google Scholar]
- Challenging diagnosis of resistance to thyroid hormone in a patient with pituitary adenoma. BMJ Case Reports. 2019;12:e229430.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone identification of thirteen novel mutations in the thyroid hormone receptor beta gene. J Clin Invest. 1994;94:506-15.
- [CrossRef] [PubMed] [Google Scholar]
- Human genetics of thyroid hormone receptor beta: Resistance to thyroid hormone beta (RTHâ) Methods Mol Biol. 2018;1801:225-40.
- [CrossRef] [PubMed] [Google Scholar]
- Mutational landscape of resistance to thyroid hormone beta (RTHâ) Mol Diagn Ther. 2019;23:353-68.
- [CrossRef] [PubMed] [Google Scholar]




