Translate this page into:
Tumour-induced osteomalacia
Correspondence to ATHIRA RAMAKRISHANAN; docathira.r@gmail.com
[To cite: Ramakrishanan A, Parekh A, Gayana S, Velusamy S, Sadhoo A. Tumour-induced osteomalacia. Natl Med J India 2024;37:253–6. DOI: 10.25259/NMJI_639_21]
Abstract
Tumour-induced osteomalacia (TIO) is a rare paraneoplastic syndrome caused by increased production of fibroblast growth factor 23 (FGF-23). A 45-year-old man presented to us with progressive weakness over 2 years along with recurrent fractures with minimal trauma. His FGF-23 was found to be above the normal range and DOTATATE positron emission tomography (PET) scan showed a well-defined enhancing soft tissue density involving the left posterior ethmoid with extension to the spheno-ethmoidal recess and sphenoid sinus ostium. He underwent endoscopic sinus surgery and excision of the tumour. Histopathological examination showed features of phosphaturic mesenchymal tumour–mixed connective tissue type. Postoperatively the serum phosphorus level increased from day 1 and reached the normal value of 2.5 mg/dl on day 3. He was discharged and continued on oral calcium and vitamin D3. Gradually his myalgia improved and he started walking independently over the next 1 month. The mean delay from symptom onset to treatment in our patient was 2 years and 5 months. Timely diagnosis and meticulous follow-up are necessary for the management of patients with this rare disorder.
INTRODUCTION
Tumour-induced osteomalacia (TIO), otherwise known as oncogenic osteomalacia, is a rare paraneoplastic syndrome caused by increased production of fibroblast growth factor 23 (FGF-23).1,2 FGF-23 acts primarily at the proximal renal tubule to inhibit phosphate reabsorption and 1α-hydroxylation of 25-hydroxyvitamin D, which leads to hypophosphataemia due to renal phosphate wasting, eventually leading to skeletal under mineralization and osteomalacia.3,4 Patients diagnosed with TIO are most often adults who report long-standing, progressive muscle and bone pain, weakness and fatigue that often predate the recurrent fractures5,6 associated with persistent hypophosphataemia due to renal phosphate wasting.7 The blood phosphate level is not assessed as a matter of routine and hence hypophosphataemia is commonly missed unless examined by a nephrologist or endocrinologist. The diagnosis of TIO and identifying the responsible tumours can be a challenge due to the small size of the tumour often leading to its misdiagnosis or remaining undetected.8,9 These tumours may not be visible in contrast CT scan or even PET imaging and may require DOTATATE PET scan to confirm their presence. The standard treatment for TIO is surgical removal of a tumour with a wide margin ensuring complete resection, as recurrence has been reported in literature.8–10 Delayed diagnosis of TIO can lead to a major decrease in quality of life and functional impairment.1 We describe a 45-year-old man with TIO who was diagnosed and managed at our tertiary referral centre in southern India.
THE CASE
A 45-year-old man presented to our institution with a 2-year history of progressive weakness and recurrent fractures with minimal trauma. He was found to have hairline fractures involving the radius, femur and calcaneum. The patient underwent a fluorodeoxyglucose (FDG)-PET scan in July 2019 showed evidence of sinusitis in the post-ethmoid and sphenoid recess region. There was non-specific enhancement without demarcation of a soft tissue tumour. He was diagnosed with osteomalacia. However, no focal lesions were discovered which are usually causative for osteomalacia. A conservative treatment regimen was initiated with oral calcium, calcitriol and bisphosphonates, but there was no improvement in the patient’s symptoms. The patient continued to suffer from severe myalgia and muscle weakness and gradually became wheelchair bound.
During the present admission, he was examined by a nephrologist who repeated the laboratory examination for serum calcium, electrolyte levels and other biochemical parameters. Laboratory examination revealed high serum alkaline phosphatase (334 U/L) and low phosphorous levels (1 mg/dl; Table I).
| Parameter | Value |
|---|---|
| Sodium | 141 mmol/L |
| Potassium | 4.27 mmol/L |
| Chloride | 106 mmol/L |
| Bicarbonate | 25.3 mmol/L |
| Immunoglobulin A | 2.57 g/L |
| Immunoglobulin G | 14.0 g/L |
| Immunoglobulin M | 0.73 g/L |
| C-reactive protein | 2.6 mg/L |
| Lactate dehydrogenase | 178 U/L |
| Glycosylated haemoglobin | 5.4% |
| Parathyroid hormone (intact) | 53.1 pg/ml |
| Rheumatoid arthritis factor | Negative |
| Haemoglobin | 13.4 g/dL |
| Alkaline phosphatase | 334 U/L |
| Erythrocyte sedimentation rate | 7 mm in first hour |
| Phosphorus | 1.0 mg/dl |
| Calcium | 8.6 mg/dl |
| Creatinine kinase | 164 U/L |
| Creatinine | 0.8 mg/dl |
| 24-hour urine phosphorous | 613 mg/dl |
| Uric acid | 4.5 mg/dl |
A DOTATATE PET scan was done which showed a well-defined, enhancing, soft tissue density involving the left posterior ethmoid with extension to the sphenoethmoidal recess and sphenoid sinus ostium. No other lesion was seen on the DOTATATE scan (Fig. 1). Based on this diagnosis, the patient underwent endoscopic sinus surgery and excision of the tumour. A soft tissue lesion was involving the posterior ethmoid sinus and sphenoid sinus ostium and was removed completely.
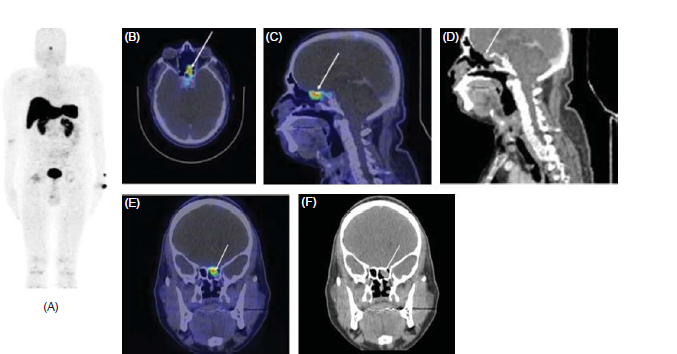
- (A) Whole body Ga-68 DOTANOC PET CT images (B) fused axial, (C) fused sagittal, (D) Sagittal, (E) fused coronal and (F) coronal CT image, showing a soft tissue density measuring 8×21×17 mm with Max SUV 5.5.
The patient showed gradual improvement in serum phosphorus level from day 1 after surgery which reached to normal range of 2.5 mg/dl on day 3. The FGF-23 levels also returned to normal.
Histopathology of the lesion showed fragments of a polyp lined by ciliated pseudostratified columnar epithelium with an underlying neoplasm composed of spindle shaped cells arranged in sheets. The tumour cells showed ill-defined cell borders, moderate eosinophilic cytoplasm, plump oval nuclei with fine chromatin and inconspicuous nucleoli. The mitotic rate was 2/10 high power fields. There was no evidence of necrosis or nuclear atypia or hypercellular areas. The intervening stroma showed focal myxoid areas and many thick and thin-walled slit-like vascular spaces resembling a haemangiopericytoma. (Fig. 2). Immunohistochemistry showed positive staining for vimentin (Figs 3 and 4) and negative staining for CD34, Desmin, S100, smooth muscle actin (SMA), Synaptophysin, Chromogranin A and STAT6. The Ki67 proliferation index was 4%. The tumour did not invade the bone and there were no pathological changes suggesting malignancy. The phosphorous level was monitored daily for the first postoperative week and thereafter once in a week. The patient was discharged and continued on oral calcium and vitamin D3. His myalgia improved and he started walking independently over the next 1 month. Mean delay from the time of onset of symptoms to diagnosis and treatment in our patient was 2 years and 5 months.
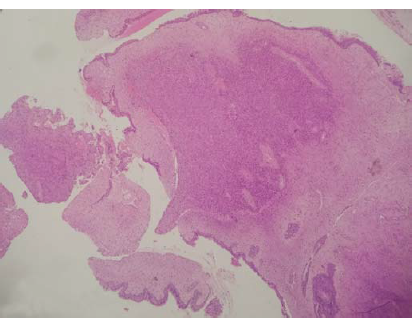
- Polypoidal lesion displaying a well-defined distinct cellular neoplasm arranged in sheets (40× view, H&E).
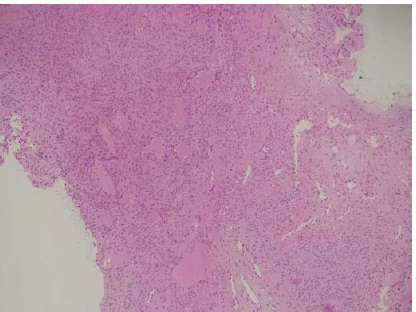
- Spindle cell tumour with prominent slit-like and branching vascular spaces (100×, H&E).
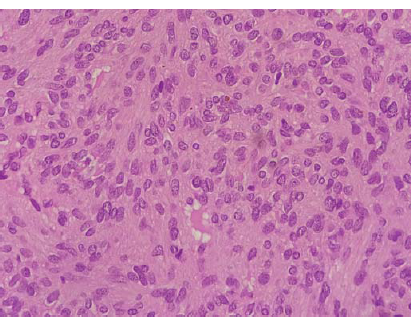
- Tumour cells show moderate eosinophilic cytoplasm, plump oval nuclei and inconspicuous nucleoli (400×, H&E).
DISCUSSION
Approximately 500 cases of TIO have been reported in the literature worldwide, among which about 40 cases are from India and about 11 cases are from southern India. In India >90% of the cases have been reported in the past 10 years of which about half have been reported after 2015, indicating a growing recognition of this disease. However, data on the true prevalence and incidence of TIO is lacking.
The mean age at diagnosis of TIO is 40–45 years with a range of 25–65 years, including few cases in children (<10 years) and the elderly (>70 years) with well-balanced distribution between genders. This is consistent with findings reported from China.9,10 In our case, the age of the patient was 45 years at the time of initial presentation which is similar to the previous reported studies in southern India.11–16 This implies that TIO usually occurs among patients in their early 40s in our population.
Due to difficulty in diagnosis of the disease and finding the tumour location, the average length of time from onset of symptoms to a correct diagnosis is usually long and often exceeds 3 years, while definitive treatment is further delayed by an average of 5 years.4,11 Most patients with TIO are often misdiagnosed for various other causes of hypophosphataemia.17 In our patient, the time between onset of symptoms to diagnosis and treatment was 2.5 years which is similar to the previous reports.11–16,18
The diagnostic assays done for diagnosis of TIO consist of fasting serum phosphorus; serum calcium, alkaline phosphatase, creatinine, intact parathyroid hormone (PTH), serum 1,25-dihydroxy vitamin D (calcitriol) and fasting 2-hour urine phosphorus, creatinine, calcium, amino acids and glucose.5 The best way to assess phosphate homeostasis is by calculating the maximum tubular resorption of phosphorus factored for glomerular filtration rate (TmP/GFR).4 Refractory hypophosphataemia despite correction of hypovitaminosis D should be investigated for TIO. Patients with refractory hypophosphataemia, normocalcaemia, elevated alkaline phosphatase and low TmP/GFR should have FGF-23 levels measured. Patients with elevated levels of FGF-23 should undergo imaging for localization.1,2 Until the causative tumour is identified the diagnosis of TIO is presumptive and other renal phosphate-wasting syndromes should be considered. Usually in patients with TIO, a family history of hypophosphataemia and bone disorders is absent and onset and severity of symptoms are more acute than in other phosphate wasting disorders as in our patient.
Recent studies have shown the reliability of total FGF-23 assays in the diagnosis and management of TIO and to confirm whether tumours have been completely removed by surgery.20 However, due to limited availability, FGF-23 levels are estimated by C-terminal ELISA technique that measures both intact and C-terminal fragments of FGF-23.21 With the half-life of FGF-23 being approximately an hour, FGF-23 levels fall drastically post-surgery.22
The majority of patients with TIO are due to phosphaturic mesenchymal tumour (mixed connective tissue variant), a rare tumour that is found mainly in soft tissues and bone in lower limbs as small and clinically inapparent lesions. Nearly 70–80% of tumours in TIO are primitive mesenchymal tumours.11 In one of the largest studies of osteomalacia associated mesen-chymal tumours by Folpe et al.,23 phosphaturic mesenchymal tumour forms a distinct entity associated with osteomalacia and is frequently misdiagnosed as other mesenchymal tumours. This tumour is characterised by small spindle to stellate cells with normochromatic nuclei and inconspicuous nucleoli. Stroma may be myxoid or chondromyxoid with grungy or flocculent calcification. Malignancy is identified by high cellularity, high nuclear grade and mitosis of >5/10 high power fields. Identification of this tumour requires awareness of the entity, exclusion of other differentials with appropriate immunohisto-chemistry (IHC) and clinical correlation. Detection of FGF-23 in the tissue with IHC and reverse transcriptase polymerase chain reaction has been attempted, but could not be done in our patient.
The most productive functional imaging to detect TIO is somatostatin receptor-based scintigraphy (68Ga-DOTATATE/DOTANOC, Tc-HYNIC-TOC). It has a better sensitivity, specificity, positive and negative predictive value than FDG-PET.4 Anatomical tumour localization can be complemented by CT scan or MRI as per the site of tumour to facilitate surgical planning. In our patient, a CT scan was done to demarcate the bony details. Access to somatostatin receptor scintigraphy and FGF-23 estimation have increased the detection of TIO in refractory hypophosphatemia. As these tumours are very rare and the index of suspicion is low, most cases may remain undetected till the patient reaches a tertiary care hospital. We used FGF-23 assay, FDG PET and DOTATATE PET for diagnosis of TIO in our patient. Similar to us, most of the case studies reported from southern India used FGF-23 assay, DOTANOC PET CT scan and MRI for the diagnosis of TIO.11–15,18
Consistent with standard treatment followed for TIO, our patient underwent endoscopic sinus surgery and excision of the tumour. There was gradual improvement in serum phosphorus level from day 1 after surgery and the patient started walking independently within 1 month after surgery. Similarly, all the cases from southern India showed rapid improvement soon after surgical resection of the tumour.11–15,18 Tumour resection is almost always curative, and following complete resection of the tumour, there is relatively rapid improvement in symptoms as reported previously.1,11–15,18 The majority of patients demonstrate surgical cure, as observed by the return of serum phosphate to normal, by post-operative day 5 whereas in our case improvement was seen from next day after surgery. Some patients may take as long as 10 days. Further, most patients show visible improvement within days to weeks of tumour excision. Bone healing starts immediately after surgery, but depending on the severity of the disease, definitive clinical improvement may take up to 1 year or more in some patients.
Conclusion
TIO is a rare paraneoplastic syndrome. Hence clinicians and pathologists must be aware of the clinical symptoms, laboratory abnormalities and pathological features. Diagnosed early it can have good outcomes.
ACKNOWLEDGEMENTS
We thank Dr Rajasekhar Kathalagere, Orthopaedic surgeon, Fortis Hospital, Bengaluru, for facilitating patient support.
Conflicts of interest
None declared
References
- Tumor-induced osteomalacia. Calcif Tissue Int. 2021;108:128-42.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour-induced osteomalacia. Nat Rev Dis Primers. 2017;3:17044.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53-77.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia. Osteoporos Sarcopenia. 2018;4:119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia: Experience from three tertiary care centers in India. Endocr Connect. 2019;8:266-76.
- [CrossRef] [PubMed] [Google Scholar]
- The foot that broke both hips: A case report and literature review of tumor-induced osteomalacia. Case Rep Rheumatol. 2017;2017:3191673.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour-induced osteomalacia: A literature review and a case report. World J Surg Oncol. 2016;14:4.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of tumor-induced osteomalacia: A retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord. 2015;16:43.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia: An important cause of adult-onset hypophosphatemic osteomalacia in China: Report of 39 cases and review of the literature. J Bone Miner Res. 2012;27:1967-75.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-induced osteomalacia: A Sherlock Holmes approach to diagnosis and management. Ann Maxillofac Surg. 2017;7:143-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor(s) induced osteomalacia—a curious case of double trouble. J Clin Endocrinol Metab. 2014;99:395-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treating osteoporosis: A near miss in an unusual case of FGF-23 mediated bone loss. BMJ Case Rep. 2019;12:e228375.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic osteomalacia: An approach to diagnosis with a case report. J Clin Diagn Res. 2017;11:ED05-ED07.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic osteomalacia diagnosed by blood pool scintigraphy. Indian J Nucl Med. 2011;26:188-91.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic osteomalacia in a patient with an ethmoid sinus tumour. J Laryngol Otol. 2010;124:799-803.
- [CrossRef] [PubMed] [Google Scholar]
- Oncogenic osteomalacia from nasal cavity giant cell tumor. Head Neck. 2012;34:454-7.
- [CrossRef] [PubMed] [Google Scholar]
- Otolaryngologist’s encounter with oncogenic osteomalacia. Ear Nose Throat J. 2020;101:NP4-NP5.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055-61.
- [CrossRef] [PubMed] [Google Scholar]
- C-terminal to intact fibroblast growth factor 23 ratio in relation to estimated glomerular filtration rate in elderly population. KBR. 2016;41:519-26.
- [CrossRef] [PubMed] [Google Scholar]
- Octreotide scanning in the detection of a mesenchymal tumour in the pubic symphysis causing hypophosphataemic osteomalacia. Int Orthop. 2002;26:61-2.
- [CrossRef] [PubMed] [Google Scholar]
- Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: An analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1-30.
- [CrossRef] [PubMed] [Google Scholar]




