Translate this page into:
Prevalence of hearing loss in India
Correspondence to ALOK THAKAR; drathakar@gmail.com
To cite: Verma RR, Konkimalla A, Thakar A, Sikka K, Singh AC, Khanna T. Prevalence of hearing loss in India. Natl Med J India 2021;34:216–22.
Abstract
Background
Despite abundant literature, a clear and coherent understanding of hearing loss (HL) in India is limited by the wide disparity in studies.
Methods
We did a review of published peer-reviewed journal articles. Studies reporting the prevalence and degree of HL in India from 1980 to 2020 were included. Information was gathered on the population characteristics, methodology applied and the prevalence of hearing impairment. The data were analysed to identify trends and at-risk sections of population in various categories.
Results
Four hundred and forty studies were identified after a database search; 29 full-length articles were selected for final analysis. Using a 3-step screening protocol, hearing impairment (abnormal auditory brainstem response/auditory steady state response) in neonates ranged between 1.59 and 8.8 per 1000 births. Among ‘at risk’ neonates, it ranged from 7 to 49.18 per 1000 births. In children the prevalence of HL was 6.6% to 16.47%. Otitis media was the most common cause of HL in children. Community-based studies (all ages) reported prevalence of HL between 6% and 26.9% and prevalence of disabling HL between 4.5% and 18.3%. Rural areas and elderly showed a higher prevalence of hearing impairment.
Conclusion
Despite India’s improving health indices, hearing disability remains persistently high. It is a major contributor to the loss of personal potential and a financial strain for the individual and the country. A large-scale multicentric study to identify the degree and type of HL, social awareness campaigns, widespread neonatal screening, strengthening treatment facilities and well-funded rehabilitation programmes can counter the rising prevalence of hearing impairment.
INTRODUCTION
Helen Keller famously said, ‘Blindness cuts us off from things, deafness cut us off from people’. Hearing loss (HL) limits one from interacting with one’s peers, and so impacts the ability to participate in social and workplace interactions. It limits learning and professional activities. In children, it interferes with learning. In older people, deafness is linked to cognitive decline, increasing the risk of depression and dementia.
MAGNITUDE OF THE PROBLEM
Global perspective
The WHO estimates that unaddressed HL costs countries an estimated $750–790 billion annually in direct health costs and loss of productivity.1 The Global Burden of Disease study estimated that the prevalence of HL rose from 1.2 billion (17.2%) in 2008 to 1.4 billion (18.7%) in 2017.2 The WHO ranked HL as the third most common cause of years lost due to disability, contributing over 39.5 million years of healthy life lost, an increase from 27 million in 2000.3
Disabling HL (DHL) refers to bilateral moderate HL or worse. The audiological criteria are HL in the better ear of >40 dB for adults and >30 dB for children.4 In 2018, the WHO estimated that the global burden of DHL was 466 million (6.12% of the world’s population). One-third of the population above 65 years of age was suffering from DHL. The number of DHL patients is predicted to be 630 million by 2030 and 900 million by 2050.5
Indian perspective
In 1997, the WHO reported a 6.3% prevalence of DHL in India.6 It increased from 76.5 million in 2008 to 100 million in 2018. By 2018, the South Asia region (Afghanistan, Bangladesh, Bhutan, India, Nepal and Pakistan) contributed 28.2% to the global burden of DHL, an increase from 27% in 2012; 7.37% of the population, including 2.4% of all children in this region suffer from DHL compared to 4.57% and 0.5%, respectively, in high-income countries.
The 2002 National Sample Survey (NSS) found HL to be the second most common cause of disability and top cause of sensory deficit; 291 per 100 000 persons surveyed had HL, which included mainly profound (32%) and severe HL (39%).7 The 2011 Indian Census noted that 2.21% of the Indian population was afflicted with some disability. The three most common were locomotor (20%), vision (19%) and hearing (19%).8 The 75th National Sample Survey (NSS) (2017–2018) report, defined hearing disability as a difficulty in hearing dayto-day conversational speech but excluded unilateral hearing impairment. Hearing disability was estimated in 0.3% of the population. About 49.8% of them reported hearing only loud sounds or inability to hear at all.9
Collection of hearing disability data by a self-reporting strategy, while simple and efficient, is limited by its poor sensitivity in the identification of mild and moderate HL. The Census and NSS data are important in highlighting that hearing disability is among the most common disabilities experienced by the Indian population.
The National Programme for Prevention and Control of Deafness (NPPCD) was launched as a national programme in 2008. Its goal is to eliminate preventable deafness, reduce the burden of deafness to <1% and empower the hearing-impaired to lead an economically and socially productive life, by 2030.10 To achieve this, we need to understand the frequency, type and distribution of HL in the Indian population.
DHL prevalence has shown a rapidly increasing trend due to three reasons: (i) an ageing population; (ii) better identification; and (iii) excessive exposure to noise. Rosen noted that the tribal population in Sudan with no noise-making traditions of drum beating, no exposure to the mechanical noises of the modern age, and a largely vegetarian and fish-based diet experienced little HL till an advanced age.11 It is estimated that 1.1 billion young people in the world are placing their hearing at risk by unsafe listening practices while the awareness of harmful effects of noise are either not known or ignored.
The trends indicate the gravity of the problem at hand and projections for the future. It is imperative that the magnitude of HL be estimated, and targeted plans be created, catering to the needs of the hearing-impaired population. Despite multiple studies, an accurate prediction has been difficult due to differences in subject populations, case definitions, and the variability of geographical areas and periods of study.
METHODS
A systematic review of databases (PubMed and Web of Science) was done according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 To identify studies for inclusion, search strategies were created for the databases. They were searched for publication dates between 1980 and 2020, and additional searches on Google Scholar were done using a combination of subject headings and keywords: Hearing loss, Hearing impairment, Deafness, Ear disease, Otitis media, Screening, Disability and India.
Titles and abstracts of the search results were screened. Duplicate records and studies that did not measure the prevalence of HL were excluded. Studies conducted among subjects in controlled cohorts such as occupational noise exposure and congenital disorders were excluded. Full-text manuscripts of the remaining articles dealing with quantitative analysis, irrespective of the method of measurement, of hearing impairment in India at a population group, city, district, state or multi-city level, from 1980 to 2020 were retrieved. Data extraction was done by two authors (AK and RRV). Data extracted included author, year of publication, study design, study characteristics, cohort demographics, method of assessment and frequency of hearing loss. Consensus was obtained on all the points. The articles were also checked independently for consistency by two authors (AK and RRV). Any disagreements were resolved by discussion with a third author (AT).
Studies that did not define the study population characteristics or the methodology applied, or revealed any inconsistencies in the results, were excluded from the analysis.
Data analysis
The studies were broadly categorized into neonatal, childhood and community, based on the study population. We extracted information on the study year, population, methodology and the prevalence of hearing impairment. Categorized into the study populations, the data were analysed to provide a summary of the results. We endeavoured to identify a trend in the HL and DHL prevalence based on the year of the study, and by comparing age groups and rural versus urban populations. The heterogeneity in the studies with regard to testing methodology and varying case definitions was a major challenge in the overall interpretation and comparison of these studies.
RESULTS
Of 440 articles identified on the database search, 92 were removed for duplication and 348 were screened. After exclusions, 29 peer-reviewed articles were included (Fig. 1).
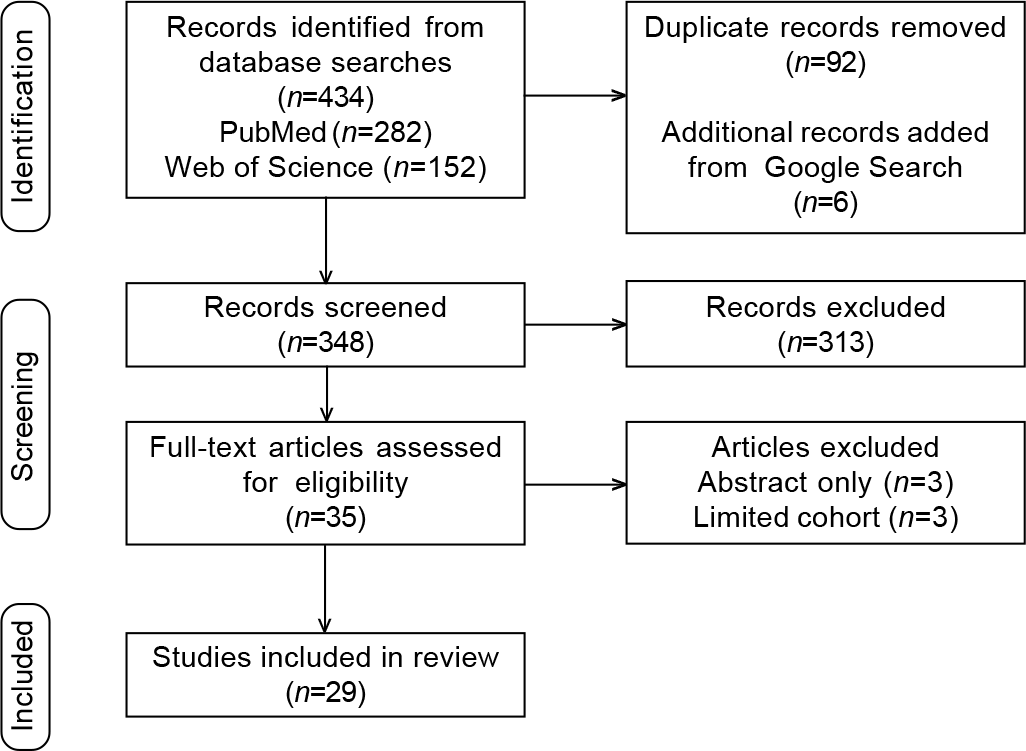
- Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram for study screening, selection, exclusion and inclusion
HL prevalence studies in India
Estimates of hearing disability from India, which have used an objective strategy to enumerate hearing disability are summarized. We categorized the studies into newborn screening population studies (Table I), paediatric population studies (Table II), and community-based studies (Table III).
| Author (year) | Method of assessment of HL Screening test → Confirmatory test | Total number | HL prevalence per 1000 | HL prevalence (well babies) per 1000 | HL prevalence (at-risk babies) per 1000 |
|---|---|---|---|---|---|
| Nagapoornima et al. (2007)13 | 2 × OAE → ABR | 1769 | 5.65 | 4.7 | 10.75 |
| John et al. (2009)14 | 2 × OAE → ABR | 500 | 6 | 2.2 | 43.5 |
| Rai and Thakur (2013)15 | 2 × OAE → ABR | 500 | 8 | 2.27 | 49.18 |
| Augustine et al. (2014)16 | 2 × AABR → ASSR | 9448 | 4.1 | – | – |
| Vignesh et al. (2015)17 | OAE → AABR | 1405 | 22.1 | 11.1 | 47.4 |
| Gupta et al. (2015)18 | 2 × AABR → conventional ABR | 2265 | 2.2 | – | – |
| Paul (2016)19 | 2 × OAE → ABR | 101 688 | 1.59 | 0.6 | 7 |
| Sachdeva and Sao (2017)20 | OAE + BOA → OAE → ABR | 2254 | 8.8 | – | – |
| Dar et al. (2017)21 | 2 × OAE → ABR | 1720 | 5.3 | – | – |
| Parab et al. (2018)22 | 2 × OAE → ABR | 8192 | 3.54 | 1.69 | 10.69 |
OAE otoacoustic emission ABR auditory brainstem response ASSR auditory steady-state response AABR automated ABR BOA behavioural observation audiometry
| Author (year) | Study area | n | Age (years) | HL (%) | HL assessment | Most common cause of HL | CHL (%) |
|---|---|---|---|---|---|---|---|
| Verma et al. (1995)23 | Rural | 613 | 5–15 | 11.97 | T F T | CSOM (69%) | – |
| Kalpana and Chamyal (1997)24 | Urban+ | 1200 | 4–17 | 1 1 | PTA; HL not defined | CSOM (43.18%) | 96.22 |
| semi-urban | |||||||
| Jacob et al. (1997)25 | Rural | 284 | 6–10 | 11.9 | PTA; HL ≥40 dB loss | CSOM (50%) | 91.18 |
| Mann et al. (1998)26 | Rural+ | 1670 | 12–14 | 16.47 | PTA; HL ≥25 dB loss | OME | – |
| urban | (urban: 6.3) | ||||||
| (rural: 32.8) | |||||||
| Das et al. (1999)27 | Tribal | 6674 | <12 | 6.62 | PTA; HL not defined | CSOM (51.83%) | 98.64 |
| Rao et al. (2002)28 | Rural | 855 | ≥5 | 11.9 | PTA; HL ≥25 dB loss | Wax (86.3%) | 81.6 |
| Arora et al. (2018)29 | Rural+ | 3964 | 2 – 9 | 3.03 | OAE; HL ≥35 dB loss | – | – |
| urban | |||||||
| Rathnaraajan et al. (2019)30 | Rural+ | 1470 | 6–14 | 35.1 | T F T | – | – |
| urban | |||||||
| Chakrabarti and Ghosh (2019)31 | Rural+ | 10 763 | 6–14 | 0.058 | PTA; HL: severe ≥70 dB, | – | NA |
| urban | (special | (severe to | profound ≥90 dB | ||||
| needs) | profound) |
CHL conductive hearing loss TFT tuning fork tests PTA pure-tone audiometry OAE otoacoustic emission NA not available CSOM chronic suppurative otitis media OME otitis media with effusion
| Author (year) | Sample size | Methodology | Findings |
|---|---|---|---|
| Singh et al. (1980)32 | 904 people | PTA | HL prevalence: ≥26 dB 7.3%; ≥41 dB 5.6%; ≥61 dB 3.5% |
| ICMR (1983)33 | 1200 households | Household survey; TFT and PTA | Prevalence: 10.7% rural; 6.8% urban; 82% bilateral; 24.4% severe; 47.9% conductive and 40.2% SNHL |
| Mishra et al. (2011)34 | 14 650 people | EARFORM software of WHO | HL rural 15.1%; urban 5.9%; DHL in <10 years of age 1.2% urban; 5.4% rural |
| Deepthi and Kasthuri (2012)35 | 175 elderly people | Self-reporting, shortened hearing handicap inventory for elderly, PTA | Sensitivity and specificity of self-reporting: For mild HL 30.9% and 93.9%; for severe HL 76.2% and 83.1% |
| Guleria et al. (2017)36 | 306 people | Structured questionnaire, PTA | HL 13.1%; 57.5% were >60 years; 70% SNHL; presbycusis 57.5%; infectious 27.5% |
| Guleria et al. (2017)37 | 306 people | Structured questionnaire, clinical evaluation, and audiological tests | HL 16.7%; 52.9% were ≥60 years; 68.6% SNHL; presbycusis 52.9%; infectious 33.3% |
| Bright et al. (2019)38 | 3573 people | 2-stage: 1st OAE →PTA (>4 years) or 2nd OAE (≤4 years) | HL 8.9%; DHL 4.5% (0.4% in 4-17 years v. 34.7% in >65 years) |
| Garg et al. (2018)39 | 664 people | WHO questionnaire, ear examination, TFT →OAE in <5 years and PTA in ≥5 years | HL 26.9%; 37.5% in 40-59 years and 39.3% in >60 years |
| Garg et al. (2018)40 | 664 people | Ear examination → OAE in <5 years and PTA in ≥5 years | HL 25.1% (32.5% rural, 26.1% urban); severe HL 51.2% |
| Khan et al. (2018)41 | 422 people | TFT | HL 23.1% (24.8% rural, 20.5% urban); >70 years of age 66.6% |
PTA pure tone audiometry TFT tuning fork tests WHO World Health Organization OAE otoacoustic emission HL hearing loss SNHL sensorineural hearing loss DHL disabling hearing loss ICMR Indian Council of Medical Research
Neonatal screening
Ten studies were included. Hearing impairment in neonates ranged from 1.59 to 8.8 per 1000 screened in studies where auditory brainstem response audiometry (ABR) was done after failing otoacoustic emission (OAE) twice.13–15,19–22 Automated ABR (AABR) double screening, followed by a conventional ABR or auditory steady state response (ASSR) audiometry showed a similar frequency of 2.2–4.1 per 1000.16,18 A single OAE followed by ABR showed a much higher failure rate of 22.1/ 1000, falling to 1.42/1000 bilateral HL after a complete audiological assessment.17 A two-step screening test followed by a confirmatory test provided consistent results.
Hearing impairment in neonates with risk factors ranged from 7 to 49.18 per 1000.13–15,19,22 Neonatal intensive care unit admissions, mechanical ventilation, low birth weight, prematurity, hyperbilirubinaemia, congenital anomalies or syndromes and family history of HL were the most common factors used to identify at-risk infants. Dar et al.21 noted that cytomegalovirus causes a 20-fold increase in early onset or congenital HL. In those without risk factors, the HL ranged from 0.6 to 4.7 per 1000 neonates.13–15,19,22 Studies with the smallest sample size reported the highest prevalence of HL.14,15
Dropouts between testing leads to underestimation of the problem and variability of the results. These were recorded as high as 17.4% between the first two screening tests and up to 72% between the screening and the confirmatory test.16 At-risk neonates had a higher dropout rate between the two screening tests but a lower one for confirmatory testing19,22 (Table IV).
| Author (year) | Total (%) | Well babies (%) | ‘At-risk’ babies (%) |
|---|---|---|---|
| Dropout rates between first and second screening | |||
| Paul (2016)19 | 3.9 | 3.3 | 5 |
| Parab et al. (2018)22 | 2.4 | 1.5 | 7.6 |
| Augustine et al. (2014)16 | 17.4 | – | – |
| Gupta (et al. 2015)18 | 14.65 | – | – |
| Dar et al. (2017)21 | 7.5 | – | – |
| Between second screening and confirmatory test | |||
| Paul (2016)19 | 6.4 | 10.2 | 3.4 |
| Parab et al. (2018)22 | 15.1 | 25.2 | Nil |
| Augustine et al. (2013)16 | 72 | – | – |
| Gupta et al. (2015)18 | 39.65 | – | – |
Hearing loss in children
Nine articles were selected for assessment in this category. The criteria for inclusion and exclusion of subjects, and the methods used for measuring HL were not consistent in the included studies. Some were school-based24–26,28,30,31 while others were done in the community.23,27,29 The number of children included, age groups and population characteristics varied with every study. The criteria for assessment ranged from tuning fork tests (TFT) to pure-tone audiometry with or without impedance audiometry (IA) to OAE. The threshold to identify HL ranged from 25 to 70 dB.
Studies using TFT to define HL, reported 11.97%23 and 35.1%30 prevalence. On audiometric evaluation, children in rural areas showed a HL of 11.9% in studies by Jacob et al.25 (HL threshold=40 dB) and Rao et al.28 (HL threshold=25 dB). It was 11% in urban areas by Kalpana and Chamyal24 and 6.62% in tribal populations by Das et al.27 A comparison of rural versus urban areas found a significantly higher loss in rural children (32.8% v. 6.3%).26 Chakrabarti and Ghosh tested 10 763 children with special needs and reported 0.58 per 1000 prevalence of severe to profound (>70 dB) sensorineural HL (SNHL).31 Conductive HL (CHL) was responsible for a vast majority of HL in children.24–28 Chronic suppurative otitis media (CSOM)4,23–27 and secretory otitis media/otitis media with effusion (OME)26,30 were the most common pathologies. Not all CSOM cases resulted in HL23,25 and the most common middle ear pathology was not always the most common cause of HL.25
Community-based studies
Ten studies were included in this analysis. The prevalence of HL ranged from 6% to 26.9% and DHL ranged from 4.5% to 18.32%.32–34,36–41 The largest of these studies was conducted by the Indian Council of Medical Research (ICMR) in 1983,33 which reported a prevalence of 10.2%. Studies with a smaller sample size reported a higher prevalence.
The rural population had higher HL numbers across all studies.33,34,36,37,39–41 The difference in rural and urban areas when evaluating DHL was smaller but substantial nonetheless36,37,39,40 (Table V). Predictably, older populations had higher prevalence of hearing impairment compared to the young. A slower rise in HL till the age of 45–50 years was noticed, beyond which there was a sharp increase in prevalence. Beyond 60–65 years of age, HL was present in more than half and DHL in more than a quarter of the population35–41 (Table VI); 39.3%–57.5% of all HL sufferers were more than 60 years old. The risk of HL in a noisy work environment (>85 dB) was also significantly higher.34
| Author (year) | Subjects | HL (%) | DHL (%) | ||||
|---|---|---|---|---|---|---|---|
| Total | Rural | Urban | Total | Rural | Urban | ||
| Singh et al. (1980)32 | 904 | 7.3 | - | - | - | - | - |
| ICMR (1983)33 | 22 600 | 10.2 | 10.7 | 6.8 | - | - | - |
| Mishra et al. (2011)34 | 14 650 | 6 | 15.1 | 5.9 | - | - | - |
| Guleria et al. (2017)36-37 | 612 | 14.9 | 16.7 | 13.1 | 5.9 | 7.2 | 5.9 |
| Garg et al. (2018)39-40 | 595 | 26.9 | 32.5 | 26.1 | 18.3 | 22.1 | 18.3 |
| Khan et al. (2018)41 | 377 | 23.1 | 24.8 | 20.5 | - | - | - |
| Bright et al. (2019)38 | 3573 | 8.9 | - | - | 4.5 | - | - |
ICMR Indian Council of Medical Research
| Author (year) | HL (%) | DHL (%) | ||
|---|---|---|---|---|
| <60 years | >60 years | <60 years | >60 years | |
| Deepthi et al. (2012)35 | - | 72 | - | 25.1 |
| Guleria et al. (2017)36,37 | 7.69 | 63.3 | - | - |
| Garg et al. (2018)39,40 | 19.4 | 67 | 12.4 | 50 |
| Khan et al. (2018)41 | 19.9 | 54.3 | - | - |
| <65 years | >65 years | <65 years | >65 years | |
| Bright et al. (2019)38 | 6.3 | 52.5 | 2.7 | 34.7 |
DISCUSSION
Prevalence and aetiology of hearing loss
Variability in study populations, selection criteria and testing methodology, all contribute to the differences across various studies. Neonatal screening studies using a 3-step evaluation (two sequential screening tests and one confirmatory ABR if twice screen failed) reported prevalence rates between 1.59 and 8.8 per 1000 population. Risk factors as described before, appear to increase the risk of HL in neonates substantially. Studies with smaller sample size have reported a significantly higher prevalence. Restricting the hearing testing to simply a 2-step screening with OAE and AABR with no further ABR for confirmation led to a much higher prevalence,17 but this may be reflective of the lesser specificity and known poor positive predictive value of these tests.
Among children, the reported prevalence of HL was between 6.62% and 16.47%. CHL was responsible for most (81.6%– 98.6%) of the hearing impairment. CSOM and OME, have remained the main reasons over the past few decades, although the contribution of CSOM towards impairment of hearing has seen a decline in the twentieth century. It was interesting to note that CSOM was also prevalent in those not considered as hearing impaired. These children are also at high risk of developing HL in the future. Chadha et al. examined 3000 children and identified a significant difference in the prevalence of CSOM in slums (7.2%), rural areas (3%) and non-slum urban areas (2.6%).42 A meta-analysis from Africa showed that CSOM was the leading cause of preventable childhood HL.43 Studies from South Africa, Nigeria and Malawi44–46 found HL in the range of 13.9% to 24% and CHL was responsible for 65% to 82% of the total HL. CHL among elementary school children in Australia was 6.3% accounting for 55% of all HL.47 Among Canadian children, HL was 4.7% and CHL was <3.5%.48 The smaller contribution of CHL, particularly of CSOM, is responsible for the lower prevalence of HL in developed countries.
Community-based studies assessing all age groups yielded a HL prevalence rate between 6% and 26.9%. The two largest community studies33,34 indicate a significantly greater burden in the rural population and in the elderly. SNHL was more prevalent, even as high as 70%, in studies with a higher proportion of elderly subjects.37,38 Smaller studies reported a considerably higher prevalence of HL. Those with hearing difficulty are more inclined to get tested and older individuals are more likely to be available for testing during the day. This bias is amplified in smaller studies. Most of the studies did not adjust for confounding factors well, except for Bright et al.38 (adjusting for age and sex) and Garg et al.39,40 (adjusting for rural v. urban population). Two of the largest studies reported HL of 6% and 8.9%.35,39 Forty-two studies from 29 countries were analysed and the age-standardized hearing impairment (≥35 dB) for adults and children was 4.9% and 0.4% in high-income countries, compared to 17% and 2.2% in the South Asian region.49
The DHL prevalence in Indian studies ranged from 4.5% to 18.3%. Among those aged more than 60 years, the prevalence was 25% to 50%. This compares similarly with DHL prevalence among Ugandan adults (11.7%)50 and in the elderly in Brazil (69.6%).51 In comparison, DHL prevalence in the developed countries was much lower. DHL in the elderly in Netherlands, was 21% in men and 18% in women.52 A review of studies from Europe estimated that 30% of men and 20% of women in Europe had a HL of 30 dB or more.53
The lack of conformity in the selection of study population and the assessment tools used, makes a collective analysis difficult, but this analysis does provide an estimate of the problem at hand and provides some insights on the way forward.
Challenges and the road ahead
Till the turn of the 21st century, developing nations were often labelled as younger populations. However, by 2050, the proportion of older persons in these countries will rise to 19% and India too will have a similar demography. Though infections and middle ear disease have predominated as the cause of HL in earlier studies,32,33 the WHO South-East Asia Region Office estimates that HL due to ageing is currently the most prevalent among non-infectious causes and that is a major cause of concern.6 Recent studies (Table III) indicate that the emerging problem of HL is shifting towards the elderly and the rates of HL in this population are inordinately high (39.3% at >60 years,39 66.6% at >70 years41).
The WHO estimates that 50% of the HL in Southeast Asia is preventable and a further 30% is treatable or can be managed by aids and devices. Primary prevention by improved community sanitation, improved general health parameters, near universal coverage of vaccination (mumps, measles, rubella)54 require an overall augmentation of economic development and health-wellness facilities. Secondary prevention (early diagnosis and treatment) and tertiary prevention (disability limitation and rehabilitation)54 are however dependent on appropriate specialist services and need augmentation. The NPPCD has been in place for a few decades. We would suggest the following thrust areas which are immediately actionable and feasible, and can bring about immediate and sustained improvement:
Significant augmentation of human resource
This is the key to deliver appropriate curative and surgical services (secondary prevention) and appropriate rehabilitation services (tertiary prevention). The existing numbers are dismal. There is 1 audiologist per 500 000 people in India while the number of otorhinolaryngologists is 1 per 140 0006,55 (WHO recommendation is 1 per 25 00054). With over 120 languages spoken across the country and a large proportion of speech and audiology services being provided at private centres, there is a large shortage of personnel and infrastructure for audiology and speech rehabilitation services. Moreover, these services are not equally distributed, and rural areas are grossly underserved. Rehabilitation can be expensive and does not always provide restoration of full hearing but have nevertheless had major technological advances over the years with effective solutions currently being provided by digital hearing aids and cochlear implants
Universal newborn hearing screening
The prerequisites for screening (congenital HL being an important health problem, acceptable test is available, and effective treatment is available and accessible) are in place for effective rehabilitation and societal integration of the ‘deaf-dumb child’. Cochlear implantation for severe HL, and hearing aid amplification for moderate HL are noted as highly effective interventions. Early rehabilitation with these modalities is noted to lead to better hearing and speech, development of communication skills and integration into normal schools. Many state governments and the Central Government provide funding for cochlear implantation via the Assistance to Disabled Persons and Chief Ministers Comprehensive Health Insurance Schemes. However, the best results are seen when such rehabilitation is started early in life, and it is imperative that the availability of cochlear implantation to children with severe HL is supported by early detection to maximize its impact. Early treatment benefits language acquisition and speech considerably.
Universal newborn screening assumes immense importance in this regard. Multiple studies have proven the importance and feasibility of the neonatal screening programme, particularly in neonates who were exposed to risk factors. Universal screening has the potential to benefit 5–10 neonates for every 1000 births. Adherence to the 1-3-6 guideline for Screening-Diagnosis-Treatment has been recommended. The hearing of all infants should be screened by 1 month of age. Those who do not pass screening, should undergo a comprehensive audiological assessment at no later than 3 months of age and receive appropriate intervention by 6 months of age.56
It is also imperative that screening is not restricted to detection of the severe and profound HL alone. Children with even mild/moderate HL also need early detection and treatment as though they may develop seemingly near normal speech, they have smaller vocabularies, difficulty listening over distance or in noisy environments, and limitation of social skills, leading to limitations in their learning and educational accomplishments.48
School hearing screening
Integration of hearing screening and an effective referral programme for all identified for surgical treatment is equally important. CSOM was noted as the cause in >50% of children with HL, more so in rural and tribal areas.23,25,27 The moderate HL associated with CSOM is a silent disability. It disrupts academic development and leads to poorer scholastic achievements. This would suggest an urgent need to strengthen services towards ear care. Regular school-based health check-ups and training of teachers, social workers and school nurses to identify hearing disability can help in early detection of ear pathologies. Rehabilitation of hearing can be effectively achieved by surgery and/or a multitude of devices. To achieve these goals, a strong and efficient infrastructure, along with adequate number of health professionals dedicated to this task are required
Identification of risk factors for high prevalence of presbycusis in our population
HL in the elderly is associated with poorer global cognitive and executive functioning, verbal fluency, attention, memory and manual dexterity making it an important cause of disability.57 We note a much higher risk of DHL in the Indian elderly (Table III) compared to those in the developed world, although studies with smaller sample size tend to overestimate the prevalence and recent studies of high prevalence are relatively small. None of the studies have provided clues to identify other risk factors that may help to formulate a comprehensive prevention strategy.
There is a need to verify these figures with a larger study and more importantly identify the risk factors leading to the higher prevalence in India. Prevention is the key and needs to be undertaken immediately, as presbycusis though a slowly progressive disorder of ageing has its roots in cumulative degeneration and impairment which progresses from early adulthood. The damaging effects of noise on the cochlea are already established and education and awareness on the same needs to be communicated to the community.
Conclusion
The disability due to hearing impairment is a major contributor to the loss of personal potential and a financial strain for the individual and the country. Low-income countries such as India are particularly susceptible due to patchy availability of screening programmes for the newborns, high prevalence of chronic ear infections in the young and the lack of resources for the elderly. It should be noted that urban slums and rural areas, which have the least access to tertiary healthcare, have the highest prevalence of hearing impairment.
Up to half of those >60 years of age suffer from DHL. Ignorance regarding the problems, lack of access to facilities and financial constraints lead to delayed or no treatment, preventing the individual from becoming a contributing member of society. The Indian population, in the next few decades will age and have a population pyramid akin to those of more developed nations. An ageing population will place a much greater burden on the healthcare facilities in India.
The variations in socioeconomic status, lifestyles, living standards and education across India, also make it difficult to understand the extent of the problem and find a common solution. While a national programme has been developed, its application on the ground leaves much to be desired. Organized large-scale data collection, scientific interpretation of the data and district, state or local level action plans based on regional factors are required. Robust programmes addressing the populations at risk and a streamlined implementation with accountability from all involved parties can help avoid a crisis.
Conflicts of interest
None declared
References
- Global costs of unaddressed hearing loss and cost-effectiveness of interventions: A WHO Report 2017. 2017. Geneva: WHO; Available at https://apps.who.int/iris/bitstream/handle/10665/254659/9789241512046-eng.pdf?sequence=1 (accessed on 1 Dec 2020)
- [Google Scholar]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-858.
- [CrossRef] [Google Scholar]
- Global Health Estimates 2020: Disease burden by Cause, Age, Sex, by Country and by Region 2000-2019. 2020. Geneva: WHO; Available at www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys (accessed on 1 Dec 2020)
- [Google Scholar]
- Grades of Hearing Impairment. 2013. Geneva: WHO; Available at www.who.int/pbd/deafness/hearing_impair-ment_grades/en/. (accessed on 20 Nov 2020)
- [Google Scholar]
- Addressing the rising prevalence of hearing loss. 2018. Geneva: WHO; Available at www.who.int/pbd/deafness/estimates/en/. (accessed on 20 Nov 2020)
- [Google Scholar]
- State of hearing and ear care. 2005. Geneva: WHO Regional Office for South-East Asia; Available at http://apps.who.int/iris/bitstream/handle/10665/205911/B1466.pdf?sequence=1&ua=1 (accessed on 1 Dec 2020)
- [Google Scholar]
- NSS 58th round (July-December 2002) Report No. 485 (58/26/1) National Sample Survey Organization, Ministry of Statistics and Programme Implementation. 2003. Government of India. Available at http://mospi.nic.in/sites/default/files/publication_reports/485_final.pdf (accessed on 1 Dec 2020)
- [Google Scholar]
- Disabled persons in India: A statistical profile 2016. 2016. New Delhi: Social Statistics Division, Ministry of Statistics and Programme Implementation, Government of India; Available at http://mospi.nic.in/sites/default/files/publication_reports/Disabled_persons_in_India_2016.pdf (accessed on 1 Dec 2020)
- [Google Scholar]
- NSS 75th Round (July 2017-June 2018) Report No. 586 (75/ 25.0). National Sample Survey Organization, Ministry of Statistics and Programme Implementation, Government of India. 2020. Health in India. Available at www.mospi.gov.in/sites/default/files/publication_reports/NSS%20Report%20 no.%20586%20Health%20in%20India.pdf. (accessed on 1 Dec 2020)
- [Google Scholar]
- Multi-country assessment of national capacity to provide hearing care. 2014. Geneva: WHO; Available at www.who.int/pbd/publications/WHOReportHearingCare_Englishweb.pdf?ua=1. (accessed on 1 Dec 2020)
- [Google Scholar]
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal screening for hearing loss: Pilot study from a tertiary care centre. Indian J Otolaryngol Head Neck Surg. 2009;61:23-6.
- [CrossRef] [PubMed] [Google Scholar]
- Universal screening of newborns to detect hearing impairment-Is it necessary? Int J Pediatr Otorhinolaryngol. 2013;77:1036-41.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal hearing screening-experience from a tertiary care hospital in southern India. Indian Pediatr. 2014;51:179-83.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and referral rates in neonatal hearing screening program using two step hearing screening protocol in Chennai--A prospective study. Int J Pediatr Otorhinolaryngol. 2015;79:1745-7.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of implementing universal newborn hearing screening at a tertiary care centre from India. Indian J Pediatr. 2015;82:688-93.
- [CrossRef] [PubMed] [Google Scholar]
- Centralized newborn hearing screening in Ernakulam, Kerala, experience over a decade. Indian Pediatr. 2016;53:15-17.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of newborn hearing screening program: A hospital based study. Indian J Otolaryngol Head Neck Surg. 2017;69:194-8.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital cytomegalovirus infection and permanent hearing loss in rural North Indian children. Pediatr Infect Dis J. 2017;36:670-3.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal screening for prevalence of hearing impairment in rural areas. Indian J Otolaryngol Head Neck Surg. 2018;70:380-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of chronic suppurative otitis media and deafness in a rural area and developing an intervention strategy. Indian J Pediatr. 1995;62:725-9.
- [CrossRef] [PubMed] [Google Scholar]
- Study of prevalence and aetiology of the hearing loss amongst school going children. Indian J Otolaryngol Head Neck Surg. 1997;49:142-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing impairment and otitis media in a rural primary school in south India. Int J Pediatr Otorhinolaryngol. 1997;39:133-8.
- [CrossRef] [Google Scholar]
- Incidence of hearing impairment among rural and urban school going children: A survey. Indian J Pediatr. 1998;65:141-5.
- [CrossRef] [PubMed] [Google Scholar]
- A study of the incidence and causation of deafness among the children in the tribal population of Manipur and its prevention. Indian J Otolaryngol Head Neck Surg. 1999;51:11-15.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing impairment and ear diseases among children of school entry age in rural South India. Int J Pediatr Otorhinolaryngol. 2002;64:105-10.
- [CrossRef] [Google Scholar]
- Neurodevelopmental disorders in children aged 2-9 years: Population-based burden estimates across five regions in India. PLoS Med. 2018;15:e1002615.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of ear disease among school children in Pondicherry, South India: A cross-sectional survey. Otolaryngology (Sunnyvale). 2019;9:379.
- [Google Scholar]
- Prevalence of severe and profound sensorineural hearing loss in school children in West Bengal, India. Indian J Otolaryngol Head Neck Surg. 2019;71:1099-106.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of deafness in a rural population of Lucknow district. Indian J Public Health. 1980;24:23-31.
- [Google Scholar]
- Project Report. 1983. New Delhi: Indian Council of Medical Research and Department of Science and Technology; Available at https://hearing.icmr.org.in/diagnosis/icmr-task-force-report-1983 (accessed on 1 Dec 2020)
- [Google Scholar]
- Prevalence of hearing impairment in the district of Lucknow, India. Indian J Public Health. 2011;55:132-4.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the use of self-reported hearing loss and the Hearing Handicap Inventory for elderly among rural Indian elderly population. Arch Gerontol Geriatr. 2012;55:762-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and etiology of hearing impairment in urban area of Shimla, Himachal Pradesh, India: A cross sectional observational study. Int J Res Med Sci. 2017;5:1252-5.
- [CrossRef] [Google Scholar]
- Hearing impairment in rural area of Himalaya: Prevalence and etiology. Int J Otorhinolaryngol Head Neck Surg. 2017;3:624-7.
- [CrossRef] [Google Scholar]
- Prevalence of hearing impairment in Mahabubnagar District, Telangana State, India. Ear Hear. 2019;40:204-12.
- [CrossRef] [PubMed] [Google Scholar]
- A cross-sectional study on hearing loss using World Health Organization protocol in Delhi. Indian J Otol. 2018;24:184-9.
- [CrossRef] [Google Scholar]
- An epidemiological study on burden of hearing loss and its associated factors in Delhi, India. Ann Otol Rhinol Laryngol. 2018;127:614-19.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hearing impairment in Aligarh: A community based study. Int J Community Med Public Health. 2018;5:2926-30.
- [CrossRef] [Google Scholar]
- Comparative prevalence of otitis media in children living in urban slums, non-slum urban and rural areas of Delhi. Int J Pediatr Otorhinolaryngol. 2014;78:2271-4.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood hearing impairment and its associated factors in sub-Saharan Africa in the 21st century: A systematic review and meta-analysis. SAGE Open Med. 2020;8:2050312120919240.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing loss in preschool children from a low income South African community. Int J Pediatr Otorhinolaryngol. 2018;115:145-8.
- [CrossRef] [PubMed] [Google Scholar]
- The hearing profile of Nigerian school children. Int J Pediatr Otorhinolaryngol. 2000;55:173-9.
- [CrossRef] [Google Scholar]
- Hearing loss in HIV-infected children in Lilongwe, Malawi. PLoS One. 2016;11:e0161421.
- [CrossRef] [PubMed] [Google Scholar]
- Slight-mild sensorineural hearing loss in children: Audiometric, clinical, and risk factor profiles. Ear Hear. 2010;31:202-12.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hearing loss among a representative sample of Canadian children and adolescents, 3 to 19 years of age. Ear Hear. 2017;38:7-20.
- [CrossRef] [PubMed] [Google Scholar]
- On behalf of the global burden of disease hearing loss expert group, global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23:146-52.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-sectional survey of hearing impairment and ear disease in Uganda. J Otolaryngol Head Neck Surg. 2008;37:753-8.
- [Google Scholar]
- Prevalence of disabling hearing loss in the elderly. Adv Treat ENT Disord. 2019;3:12-13.
- [CrossRef] [Google Scholar]
- Prevalence of age-related hearing loss, including sex differences, in older adults in a large cohort study. Laryngoscope. 2017;127:725-30.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of age-related hearing loss in Europe: A review. Eur Arch Otorhinolaryngol. 2011;268:1101-7.
- [CrossRef] [PubMed] [Google Scholar]
- Deafness and its prevention--Indian scenario. Indian J Pediatr. 1997;64:801-9.
- [CrossRef] [PubMed] [Google Scholar]
- Audiology services in India. Perspect Glob Issues Commun Sci Relat Disord. 2011;1:21-6.
- [CrossRef] [Google Scholar]
- Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898-921.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing loss and cognitive decline in the general population: A prospective cohort study. J Neurol. 2021;268:860-71.
- [CrossRef] [PubMed] [Google Scholar]




