Translate this page into:
Prevalence of chronic kidney disease among severely gas-exposed survivors in Bhopal, India
Correspondence to YOGESH DAMODAR SABDE; ysabde@yahoo.com
[To cite: Soni KK, Kalyanasundaram M, Singh S, Shubham S, Sabde YD, Prakash A, et al. Prevalence of chronic kidney disease among severely gas-exposed survivors in Bhopal, India. Natl Med J India 2023;36:5–10. DOI: 10.252549/NMJI_569_20]
Abstract
Background
The survivors of the 1984 Bhopal gas disaster frequently express concern of them being at higher risk of developing chronic kidney disease (CKD) as a consequence of the long-term health effects of gas exposure. We aimed to estimate the prevalence of CKD among the survivors of severely gas-exposed cohort assembled in 1985 after the Bhopal gas disaster to study the long-term health consequences of gas exposure.
Methods
We did this cross-sectional study with a sample size of 215 systematically selected participants among the severely gas-exposed survivors in Bhopal to estimate the prevalence of CKD. Sociodemographic and relevant past medical history of the participants was obtained using a semi-structured questionnaire and their blood and urine samples were collected. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation. Those found with reduced e-GFR and proteinuria, suggestive of CKD, were further surveyed after 3 months to differentiate CKD from acute renal damage.
Results
The prevalence of CKD among the severely gas-exposed cohort survivors in Bhopal was 16.7%. Multiple logistic regression analysis revealed that body mass index and level of education were significant predictors of CKD.
Conclusion
The prevalence of CKD among the severely exposed survivors of Bhopal was at par with the national prevalence, putting at rest the apprehension of gas-exposed survivors of being at higher risk of developing CKD.
INTRODUCTION
Chronic kidney disease (CKD) has emerged as one of the leading causes of global morbidity and mortality with its global prevalence ranging from 8% to 10%.1–6 The global burden of disease study estimated a 30% increase in CKD prevalence in 2020 compared to 1990.4 Despite being a global concern, CKD disproportionately affects people from lower-middle-income countries.5 In India, the prevalence of CKD has increased to epidemic proportions and population-based studies have reported a 4%–20% prevalence of CKD in India.2,5–10
CKD in its early stages is considered as one of the major risk factors for fatal and non-fatal cardiovascular events. When it reaches its last stage, also known as end-stage renal disease (ESRD), the financial burden of treatment through dialysis and renal replacement therapy is enormous.4,5,7,10 In resource-scarce countries such as India, <10% of patients with ESRD have access to any kind of renal replacement therapy.2,10 Hence, it is appropriate that efforts are focused on prevention rather than treatment.
The Bhopal gas tragedy in December 1984, considered among the worst industrial disasters in the history of humankind, resulted in mortality of 2500–6000 and debilitating over 200 000 people, causing major morbidity and many premature deaths.11,12 Various clinical and epidemiological studies undertaken subsequently showed a higher prevalence of chronic illnesses such as pulmonary fibrosis, bronchial asthma, chronic obstructive pulmonary disease, keratopathy and corneal opacities in the exposed population.11,13,14 The Indian Council of Medical Research (ICMR) launched a long-term, population-based epidemiological study in January 1985 to assess the long-term health effects of toxic gases on a cohort of exposed people assembled according to surrogate exposure intensity, i.e. severely, moderately and mildly exposed and this cohort is still being followed up. Although animal studies show acute histopathological changes in renal epithelial cells upon exposure to methyl isocyanate,11,15 there is a dearth of population-based studies to show the extent of renal disease in the gas-exposed survivors.
Concern has been repeatedly expressed by the gas-exposed survivors and several civil society groups that the prevalence of kidney-related ailments is too high among this vulnerable group as a consequence of the ill-effects of gas exposure in 1984. To address this concern, we did a cross-sectional study to estimate the prevalence of CKD among individuals belonging to the severely gas-exposed cohort of the ongoing long-term, population-based epidemiological study in Bhopal.
METHODS
Study population
Subsequent to the gas leakage disaster on the intervening night of 2–3 December 1984, the exposed areas were classified into severe, moderate and mild categories based on the immediate mortality occurring between 3 and 6 December 1984. In January 1985, a long-term epidemiological study was initiated to study the health effects of toxic gas exposure by assembling cohorts of 80 021 exposed persons living in areas severely, moderately and mildly affected.16 The cohort is being followed up for the past 35 years. As per the 51st round of morbidity survey conducted in 2015 by the ICMR-National Institute for Research in Environmental Health (NIREH), there were 8274 severely gas-exposed survivors living in 1751 households in four localities, namely J.P. Nagar, Kazi camp, Kainchichola, and Railway Colony in Bhopal city. The present cross-sectional study was conducted during June–December 2018 involving a selected sample of consenting individuals from this severely exposed cohort.
Sample size and sampling frame
Taking the Indian population prevalence of CKD as 17.2%,7 the sample size calculated was 214 for the defined population size of 8274 with 95% confidence level and absolute precision of 5% using OpenEpi. The list of households and exposed survivors (born before 3 December 1984) covered in the 51st round of the long-term, population-based epidemiological survey was considered as the sampling frame. With household as a sampling unit, a total of 215 households were selected using systematic random sampling. From each selected household, one survivor was included in the study. If multiple survivors fulfilled the inclusion criteria in a selected household, then one of them was recruited using the lottery method.
Sociodemography and clinical examination
A team comprising a trained physician, research assistant and nurse was involved in the data collection process. Written informed consent was obtained from all the recruited participants. Participants were interviewed in their homes using a semi-structured questionnaire for sociodemographic details, comorbid illnesses such as diabetes, hypertension, arthritis and chronic renal diseases and the history of substance use such as smoking, chewing tobacco and alcohol intake. This was followed by anthropometric measurements, clinical examination and collection of biological samples, i.e. urine and blood for assessing serum creatinine, random blood sugar level and urine protein.
Anthropometric measurements such as height and weight of the participants were taken as per the standard method. Blood pressure was measured using a digital sphygmomanometer (OMRON-Automatic Blood Pressure Monitor Model HEM-7124) in the sitting position. For each individual, the average of three readings, taken at an interval of 5 minutes, was considered as the final value of blood pressure.
Collection and processing of biological samples
Urine. Mid-stream fresh sample of urine was collected in a sterile container from each participant and albumin was assessed using dipsticks (Erba-Uro-dipcheck240).
Blood. Venous blood (4–6 ml) from each subject was collected in two vacutainers under aseptic conditions. The sample collected in plain vacutainer was used for estimating creatinine and the one collected in sodium fluoride vacutainer for estimating random blood sugar. The samples were transported to the laboratory in an icebox at 2–8 °C and processed in an IDMS standardized biochemical auto-analyser (Transasia-EM 200 ) within 4–5 hours of collection.
Calculation of estimated glomerular filtration rate
A reduced glomerular filtration rate (GFR) was considered as the indicator for kidney dysfunction and increased urinary albumin excretion as an indicator of renal damage. Estimated GFR (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) study equation.17 Staging of renal disease based on the e-GFR category and categorization of proteinuria using the dipstick readings was done as per the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines.18
Definition of study variables
Participants with either sustained decrease in e-GFR (e-GFR <60 ml/minute per 1.73 m2, i.e. stage G3a and above) or proteinuria of category A2 and above (i.e. albumin-to-creatinine ratio [ACR] >30 mg/g) for at least 3 months were defined as having CKD. Participants with reduced e-GFR and proteinuria were contacted again after 3 months to reconfirm low e-GFR and high proteinuria to ensure the chronicity of kidney disease. If the decrease in e-GFR or the presence of category A2 and above proteinuria persisted at the third month of survey in the absence of reversible factors, then the respective participants were diagnosed to have CKD. Such participants were referred for further investigation and management.
Hypertension was defined as the presence of systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg on examination or self-reported history of hypertension or the use of antihypertensive medication. Participants were defined as having diabetes mellitus when random blood sugar >200 mg/dl was detected in collected blood samples along with the presence of symptoms such as polyuria, polyphagia, polydipsia and weight loss or when participants reported a history of diabetes or use of insulin or other hypoglycaemic medication.
The WHO classification of body mass index (BMI) was used to classify participants according to their BMI category, i.e. BMI <18.5 kg/m2 was classified as undernutrition, BMI between 18.5 and 24.9 was classified as normal, BMI between 25 and 29.9 was considered as overweight and BMI >30 kg/m2 was classified as obese.
Ethical approval for the study was obtained from the Institutional Ethics Committee of ICMR-NIREH, Bhopal. Participants’ confidentiality was maintained.
RESULTS
A total of 215 gas-exposed survivors belonging to the severely exposed cohort of the long-term population-based epidemiological study were assessed for the presence of CKD. The mean (SD) age of the participants was 57.5 (12.07) years with higher participation (58.6%) of females. Nearly half the participants (50.2%) belonged to the 50–65 years age group. About 54.9% of participants did not have any formal education, whereas 30.2% of participants had completed primary level education. About 18% of participants were unemployed, 50.2% were homemakers and 71. 2% were non-vegetarian. Tobacco chewing was the most common (37.2%) substance use among the study participants (Table I).
| Variable | Categories | Frequency (%) |
|---|---|---|
| Gender | Men | 89 (41.4) |
| Women | 126 (58.6) | |
| Age group (years) | 35–50 | 52 (24.2) |
| 50–65 | 108 (50.2) | |
| >65 | 55 (25.6) | |
| Marital status | Married | 201 (93.5) |
| Single | 14 (6.5) | |
| Education | Illiterate | 118 (54.9) |
| Primary | 65 (30.2) | |
| Secondary | 8 (3.7) | |
| Higher secondary | 1 7 (7.9) | |
| Graduate and above | 7 (3.3) | |
| Occupation | Unemployed | 39 (18.1) |
| Shopkeeper | 19 (8.8) | |
| Labour | 44 (20.5) | |
| Government employee | 3 (1.4) | |
| Private employee | 2 (0.9) | |
| Homemaker | 108 (50.2) | |
| Dietary habits | Vegetarian | 62 (28.8) |
| Non-vegetarian | 153 (71.2) | |
| Substance use | No substance use | 110 (51.2) |
| Smoking | 2 3 (10.7) | |
| Alcohol | 1 (0.5) | |
| Tobacco chewing | 80 (37.2) | |
| All three habits | 1 (0.5) |
Mean (SD) age 57.5 (12.07) years
The prevalence of CKD was found to be 16.7% (95% CI 12.2– 22.2). The mean (SD) e-GFR of the participants was 75.4 (17.9) with 14.4% of participants belonging to the G3a category of eGFR (Fig. 1). Proteinuria was present in 2.7% (6/215) and diabetes and hypertension were present in 20% (43/215) and 56.2% (121/215), respectively.
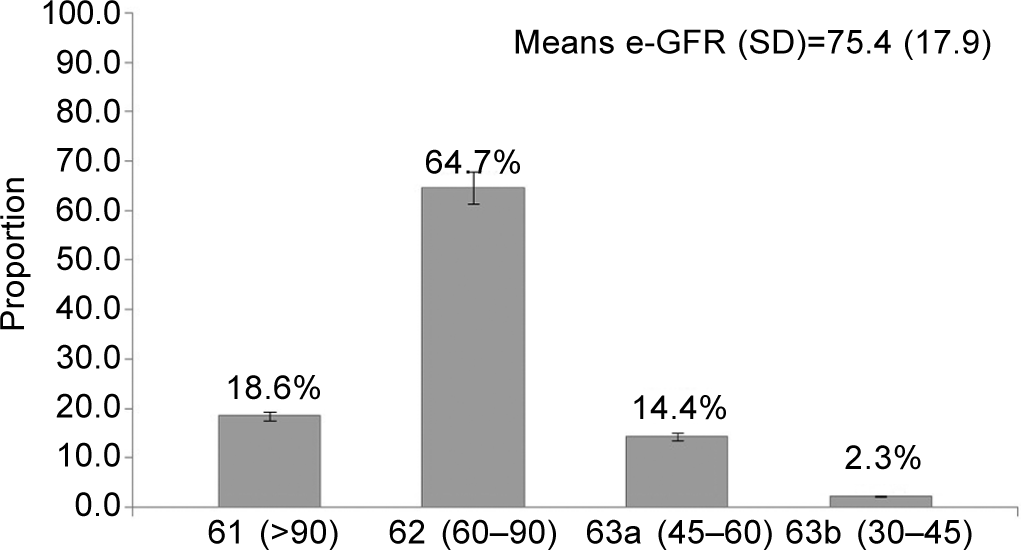
- Distribution of the study population according to their estimated glomerular filtration rate category given by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (n=215)
The mean (SD) age of the participants diagnosed as having CKD (n=36) was 64.2 (13.9) years and their mean e-GFR was 53.4 (10.9). Three-fourths (75%, 27/36) of the participants with CKD had hypertension, 30.6% (11/36) had diabetes, 27.8% (10/36) were overweight and 8.3% (3/36) were obese. Of the 36 participants with CKD, 32 did not have any history of renal disease and were newly diagnosed with CKD.
Univariate analysis revealed that factors such as gender, age, educational status, occupation, BMI and hypertension were significantly associated with CKD (Table II). To find out the individual effect of these factors on CKD after adjusting for other confounders, multivariate analysis was done. Factors with p<0.1 were included for multivariate analysis. This showed BMI and level of education to be independent predictors of CKD in our study population (Table III). Being literate reduced the risk of development of CKD by 65% (adjusted OR=0.347, 95% CI=0.13–0.93) when compared with illiterate. A high BMI increased the likelihood of development of CKD as there was a 3.6 times higher risk among overweight participants and 9.3 times higher risk among obese compared to participants with normal BMI. The R2 (Nagelkerke) value of the final model was 0.212, thereby explaining about 21.2% variation of CKD in the study population.
| Variable | CKD | Unadjusted OR (95% CI) | p value | |
|---|---|---|---|---|
| Absent, n (%) | Present, n(%) | |||
| Gender | ||||
| Men | .80 (89.9) | .09 (10.1) | Reference | |
| Women | .99 (78.6) | .27 (21.4) | 2.424 (1.08–5.45) | 0.029* |
| Age group (years) | ||||
| 35–50 | .47 (90.4) | .5 (9.6) | Reference | |
| 50–65 | .93 (86.1) | .15 (13.9) | 1.52 (0.52–4.43) | 0.45 |
| >65 | .39 (70.9) | .16 (29.1) | 3.86 (1.29–11.47) | 0.02* |
| Mean age (SD) in years† | 56.2 (11.2) | 64.2 (13.9) | 1.059 (1.03–1.09) | 0.01* |
| Education | ||||
| Illiterate | .92 (78.0) | .26 (22.0) | Reference | |
| Literate | .87 (89.7) | .10 (10.3) | 0.407 (0.18–0.89) | 0.02* |
| Occupation | ||||
| Unemployed | .116 (78.9) | .31 (21.1) | Reference | |
| Employed | .63 (92.6) | .5 (7.4) | 0.297 (0.11–0.81) | 0.01* |
| Dietary habits | ||||
| Vegetarian | .55 (88.7) | .07 (11.3) | Reference | |
| Non-vegetarian | .124 (81) | .29 (19.0) | 1.838 (0.76–4.45) | 0.17 |
| Smoking | ||||
| No | .159 (83.2) | .32 (16.8) | Reference | |
| Yes | .20 (83.3) | .4 (16.7) | 0.994 (0.32–3.11) | 0.99 |
| Tobacco (chewable) | ||||
| No | .112 (83.6) | .22 (16.4) | Reference | |
| Yes | .67 (82.7) | .14 (17.3) | 1.064 (0.51–2.22) | 0.87 |
| Body mass index | ||||
| Underweight | .34 (82.9) | .7 (17.1) | 1.493 (0.57–3.92) | |
| Normal | .116 (87.9) | .16 (12.1) | Reference | 0.42 |
| Overweight | .26 (72.2) | .10 (27.8) | 2.788 (1.13–6.84) | 0.03* |
| Obesity | .03 (50.0) | .3 (50.0) | 7.250 (1.34–39.1) | 0.02* |
| Hypertension | ||||
| No | .85 (90.4) | .9 (9.6) | Reference | |
| Yes | .94 (77.7) | .27 (22.3) | 2.713 (1.21–6.01) | 0.01* |
| Diabetes mellitus | ||||
| No | .147 (85.5) | .25 (14.5) | Reference | |
| Yes | .32 (74.4) | .11 (25.6) | 2.021 (0.91–4.52) | 0.08 |
| Variable | Adjusted OR | 95% CI | p |
|---|---|---|---|
| Gender | |||
| Men | Reference | ||
| Women | 1.425 | 0.463–4.392 | 0.54 |
| Age group (years) | |||
| 35–50 | Reference | ||
| 50–65 | 0.842 | 0.260–2.730 | 0.77 |
| >65 | 1.675 | 0.488–6.121 | 0.44 |
| Education | |||
| Illiterate | Reference | ||
| Literate | 0.347 | 0.13–0.934 | 0.04* |
| Occupation | |||
| Unemployed | Reference | ||
| Employed | 0.530 | 0.135–2.08 | 0.36 |
| Hypertension | |||
| No | Reference | ||
| Yes | 1.684 | 0.688–4.117 | 0.25 |
| Diabetes mellitus | |||
| No | Reference | ||
| Yes | 1.528 | 0.602–3.877 | 0.37 |
| Body mass index | |||
| Normal | Reference | ||
| Underweight | 0.995 | 0.349–2.833 | 0.99 |
| Overweight | 3.61 | 1.282–10.15 | 0.02* |
| Obese | 9.34 | 1.232–58.725 | 0.03* |
DISCUSSION
Studies conducted after the Bhopal gas disaster documented a high prevalence of several chronic illnesses related to the respiratory, gastrointestinal, neurological, psychiatric and ophthalmic systems.11,13,14 However, the burden of renal disease among this community remained undocumented. In the past few years, a concern has been voiced that the prevalence of kidney-related ailments is higher in the gas-exposed community compared to the general population as a consequence of the ill-effects of gas exposure. We did not include moderately and mildly exposed cohort survivors in the study primarily due to operational reasons and second, it was assumed that any long-term adverse effect of gas exposure on the renal system resulting in the development of CKD will be maximal in the severely exposed cohort and thus they will have the highest prevalence of CKD among the three cohorts.
We found a 16.7% prevalence of CKD in the severely exposed cohort of gas survivors. An earlier cross-sectional study conducted using similar case definitions and equations as used in our study, which covered 6120 subjects across various Indian cities including Bhopal reported in 2013, found a nearly similar prevalence (17.2%) of CKD among Indian adults.7 Further, Ene-Iordache et al.5 in their cross-sectional study carried out in 12 lower- and middle-income countries from six regions of the globe to assess the prevalence and awareness of CKD and its risk factors reported a 16.8% prevalence of CKD in India, which matches with the prevalence reported in our study. A current systematic review based on eight Indian studies reported a 10.2% pooled prevalence of CKD in India.2 Among the studies included in this systematic review, the highest prevalence in India was 17.2% among participants of the Screening and Early Evaluation of Kidney Disease study, which screened 6120 subjects from 13 academic and private medical centres across India7 and the lowest prevalence of 4.2% was found among <20 years old adult residents from Delhi.19 Thus, the prevalence of CKD estimated in our study was comparable with the national prevalence.
The minor variations in the reported prevalence among different studies from India could be because different definitions of CKD were used, or the difference in the methods/equations adopted in estimating GFR, the difference in the study population, as well as differences in the geographical area studied, etc.10,19–21 For instance, Agarwal and Srivastava22 reported a prevalence of CKD of 0.79% in India. The prevalence might have been underestimated due to the use of serum creatinine >1.8 mg/dl as the cut-off. On the other hand, Anupama and Uma10 reported the prevalence of CKD as 6.3% using the MDRD equation in a rural population of southern India. The difference in the CKD prevalence estimated in our study with that of other studies2,10,22 could partly be explained by the high prevalence of diabetes (20%) and hypertension (56.2%) among the subjects in our study. In 2005, Modi and Jha23 conducted a large ESRD incidence study among the gas-exposed victims of Bhopal visiting the dedicated tertiary care hospital, and reported that average crude and age-adjusted incidence rates were 151 and 232 per million population, respectively. In our community-based, cross-sectional study conducted among the severely gas-exposed cohort survivors, we found that the prevalence of later stage of CKD (G3b) was 2.3%. There is a need for future community-based prospective studies to ascertain the change in incidence rates in this population. Further, Modi and Jha in their study found that diabetic nephropathy was one of the leading causes of ESRD,23 which was reflected in our study as well.
In our study, 89% of the subjects diagnosed with CKD were unaware of the status of their renal condition and hence were new cases of CKD identified during the study. This indicates the lack of screening for renal damage among those suffering from other chronic non-communicable diseases (NCDs). Hence, there is a need for designing and implementing country-specific standard guidelines for screening of patients with NCDs for their renal function. A notable finding in our study was the lower prevalence of proteinuria (2.7%), which is at variance to earlier population-based studies on CKD.10,24 This could partly explain why a higher proportion of patients with CKD were unaware of their renal condition in our study because, at the primary care level, screening of renal function is based on urine protein analysis. This should be considered while planning any screening programme in this population.
In our study population, BMI and level of education were found to be significant independent predictors of CKD. Having some formal education significantly reduced the likelihood of development of CKD compared to illiterate individuals. Consistent with our findings, several studies in western countries24–28 and India19,29,30 reported an increased risk of CKD and its outcomes in individuals with a lower level of education. In an observational cohort of 61 457 participants of the Kidney Early Evaluation Program study, it was found that higher educational level was associated independently with a lower prevalence of CKD and lower mortality in those in the cohort who had chronic diseases including CKD.26 The educational status of an individual may impact development of CKD and its diagnosis through several factors such as health literacy and knowledge of the impact of comorbid illnesses on renal function, health-related behaviour including healthcare seeking and utilization and access to healthcare delivery systems.27 Many studies have reported the association of unhealthy behaviours such as consumption of unbalanced diet, smoking and alcohol intake with a lower educational level.26–28 Similarly, an association has also been observed between lower educational level and diseases such as diabetes and hypertension.3 A study exploring the socioeconomic disparities in prevalence of CKD revealed that education was associated more closely with the prevalence of CKD and its clinical outcomes as compared to income.31 Factors such as health behaviour, comorbid illness and health system access that are influenced by lower educational status may lead to higher risk of CKD and thus need to be studied in detail in Indian settings.
We found a dose–response relationship with BMI and CKD. Compared to individuals with normal BMI, the likelihood of developing CKD among overweight individuals was three times higher and for obese individuals, the likelihood increased to nine times. This finding is in agreement with previous studies conducted across a diverse population.32–34 Overweight and obesity result in a wide range of metabolic abnormalities, which may affect renal function.35,36 Presumably, some of the harmful effects of obesity on kidneys are mediated through comorbid illnesses such as diabetes and hypertension. Evidence exists about the independent and direct effect of adiposity on kidneys induced by the endocrine activity of adipose tissue.36,37 However, whether BMI is an appropriate indicator of adiposity is a debatable issue.37
Limitations
In our study, GFR was estimated based on the MDRD equation. However, it has been shown that the accuracy of GFR estimation could be improved using the CKD-EPI equation using cystatin C in combination with serum creatinine.38,39 We could not measure cystatin C due to resource constraints. We calculated the prevalence of CKD-based GFR estimated by CKD-EPI-Cr (creatinine alone) equation and there was minimal variation (<1%) with the prevalence calculated by the MDRD method. The mean GFR calculated by MDRD (75.29 [17.9]) and CKD EPICr (75.34 [18.2]) was similar. The MDRD equation that we used to estimate GFR has its inherent limitations. This equation possibly underestimates GFR among healthy individuals.39 Moreover, MDRD formulae have not been validated in the Indian population and no correction factor has been derived to modify the equation to suit the Indian population.10,39 However, the accuracy of the MDRD equation is widely accepted to estimate GFR based on creatinine value in population-based studies.10 We used spot urine analysis to assess the prevalence of proteinuria, which is less sensitive than ACR or AER (urine albumin excretion).2,20 Further, in our study, the assessment of renal function and damage in participants diagnosed with CKD initially was repeated after 3 months to discriminate between acute and chronic renal disease. However, around 44% (16/36) of subjects with reduced GFR and/or proteinuria refused to give consent for a second investigation when contacted after 3 months; these individuals were classified as having CKD based on the results of their initial investigation and this is a major limitation of the study. However, this limitation would have resulted in overestimation rather than underestimation. Moreover, the small sample size was a limitation in ascertaining the risk factors of CKD through logistic regression analysis, as our study did not have enough power to pick up many predictors including proven risk factors such as age, diabetes and hypertension.
Conclusions
Our findings of a 16.7% community-based prevalence of CKD among the severely gas-exposed cohort population in Bhopal is similar to the national prevalence. This should put at rest the perceived concern of higher prevalence of CKD in gas-exposed survivors. The higher prevalence of diabetes and hypertension may largely be responsible for the CKD prevalence. It would be prudent to explore the role of gas exposure, if any, in causing CKD or increasing vulnerability to CKD through a well-designed case–control study with adequate sample size. There is also a need for primary prevention programmes targeting weight reduction and increasing physical activity at the individual as well as the community level to reduce the burden of CKD and other NCDs. Further, a secondary prevention programme (early screening of renal disease in the at-risk population) with appropriate regional specific guidelines needs to be developed and implemented.
ACKNOWLEDGEMENTS
Our sincere thanks to all the study participants. We acknowledge the support of field and office staff of ICMR-NIREH in data collection and data management. We also acknowledge Dr Tanwi Trushna, Scientist B, ICMR-NIREH, for her contribution towards improvement of language of the manuscript. This research was funded by the Indian Council of Medical Research.
Conflicts of interest
None declared
References
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-88.
- [Google Scholar]
- Prevalence of chronic kidney disease in South Asia: A systematic review. BMC Nephrol. 2018;19:291.
- [CrossRef] [PubMed] [Google Scholar]
- Burden and predictors of hypertension in India: Results of SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2014;15:42.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic kidney disease. Lancet. 2020;395:662-4.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease and cardiovascular risk in six regions of the world (ISNKDDC): A cross-sectional study. Lancet Glob Health. 2016;4:e307-e319.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic kidney disease in cuttack district of Odisha, India. Int J Environ Res Public Health. 2020;17:456.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and risk factors of chronic kidney disease in India-results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int. 2015;88:178-85.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of chronic kidney disease in a semi-urban population of western India. Clin Kidney J. 2016;9:438-43.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic kidney disease among adults in a rural community in south India: Results from the kidney disease screening (KIDS) project. Indian J Nephrol. 2014;24:214-21.
- [CrossRef] [PubMed] [Google Scholar]
- Bhopal Gas Tragedy: Review of clinical and experimental findings after 25 years. Int J Occup Med Environ Health. 2009;22:193-202.
- [CrossRef] [PubMed] [Google Scholar]
- The Bhopal disaster and its aftermath: A review. Environ Health. 2005;4:6.
- [CrossRef] [PubMed] [Google Scholar]
- Annual change in spirometric parameters among patients affected in Bhopal gas disaster: A retrospective observational study. Lung India. 2013;30:103-7.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective analysis of lung function abnormalities of Bhopal gas tragedy affected population. Indian J Med Res. 2012;135:193-200.
- [Google Scholar]
- A retrospective review of cytogenetic studies on methyl isocyanate with special reference to the Bhopal gas tragedy: Is the next generation also at risk? Int J Occup Med Environ Health. 2013;26:324-36.
- [CrossRef] [PubMed] [Google Scholar]
- Annual Report 2013-2014. Bhopal: National Institute for Research in Environmental Health (Indian Council of Medical Research);
- [Google Scholar]
- Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-54.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for kidney disease guideline updating: A report by the KDIGO methods committee. Kidney Int. 2016;89:753-60.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: An observational, cross-sectional study. BMC Nephrol. 2009;10:4.
- [CrossRef] [PubMed] [Google Scholar]
- Global prevalence of chronic kidney disease--A systematic review and meta-analysis. PLOS One. 2016;11:e0158765.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease of unknown etiology: Hotspots in India and other Asian countries. Semin Nephrol. 2019;39:272-7.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease in India: Challenges and solutions. Nephron Clin Pract. 2009;111:c197-c203.
- [Google Scholar]
- The incidence of end-stage renal disease in India: A population-based study. Kidney Int. 2006;70:2131-3.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hypertension among Indian adults: Results from the great India blood pressure survey. Indian Heart J. 2019;71:309-13.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between educational and occupational levels, and Chronic Kidney Disease in a multi-ethnic sample-The HELIUS study. PLoS One. 2017;12 Available at 10.1371/journal.pone.0186460 (accessed on 25 Apr 2020)
- [CrossRef] [PubMed] [Google Scholar]
- Association of educational attainment with chronic disease and mortality: The Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;58:228-34.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of educational attainment on health outcomes in moderate to severe CKD. Am J Kidney Dis. 2016;67:31-9.
- [CrossRef] [PubMed] [Google Scholar]
- Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3:1316-23.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of early stages of chronic kidney disease in healthy army personnel. Med J Armed Forces India. 2011;67:9-14.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of early stages of chronic kidney disease in apparently healthy central government employees in India. Nephrol Dial Transplant. 2010;25:3011-17.
- [CrossRef] [PubMed] [Google Scholar]
- Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809-19.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertension and kidney function in an adult population of West Bengal, India: role of body weight, waist circumference, proteinuria and rural area living. Nephrology (Carlton). 2013;18:798-807.
- [CrossRef] [PubMed] [Google Scholar]
- Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128•9 million children, adolescents, and adults. Lancet. 2017;390:2627-42.
- [Google Scholar]
- Body mass index and preclinical kidney disease in Indian adults aged 40 years and above without chronic kidney disease. Clin Exp Nephrol. 2014;18:919-24.
- [CrossRef] [PubMed] [Google Scholar]
- Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871-80.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and kidney disease: Hidden consequences of the epidemic. Indian J Nephrol. 2017;27:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity paradox in advanced kidney disease: From bedside to the bench. Prog Cardiovasc Dis. 2018;61:168-81.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of CKD-EPI cystatin c and creatinine glomerular filtration rate estimation equations in Asian Indians. Int J Nephrol. 2014;2014:746497.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKDEPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61:57-66.
- [CrossRef] [PubMed] [Google Scholar]




