Translate this page into:
The association between Behçet disease activity and elevated systemic immune–inflammation index: A retrospective observational study in a tertiary care hospital
Correspondence to DILEK MENTESOGLU; drdilek.2013@gmail.com
[To cite: Mentesoglu D, Atakan N. The association between Behçet disease activity and elevated systemic immune–inflammation index: A retrospective observational study in a tertiary care hospital. Natl Med J India 2024;37:74–8. DOI: 10.25259/NMJI_212_2022]
Abstract
Background
The systemic immune–inflammation index (SII) is a novel marker for predicting the prognosis in patients with various diseases and cancers. We aimed to investigate the relationship between SII and disease activity in patients with Behçet disease (BD).
Methods
Our retrospective study included 513 patients with BD aged ≥18 years. The patients were classified into an active group (n=355) and an inactive group (n=158). Pearson correlation analysis was performed to elucidate correlations between the SII and other markers. Binary logistic regression analysis was used to determine BD-related risk factors. Receiver operating characteristic (ROC) curves were computed to assess cut-offs for the predictive value of the SII and other markers.
Results
Patients with active BD had a significantly higher SII (p<0.001) than those in the inactive group. ROC analysis revealed that the optimal SII cut-off value to identify BD activity was 526.23, with 70.4% sensitivity and 70.3% specificity. Pearson correlation coefficient (r) demonstrated a significant positive correlation between SII, and the C-reactive protein level (r=0.427, p<0.001), erythrocyte sedimentation rate (r=0.422, p<0.001), platelet– lymphocyte ratio (r=0.711, p<0.001), and neutrophil– lymphocyte ratio (r=0.672, p<0.001). According to binary logistic regression analysis, the SII (odds ratio [OR] 1.003; 95% confidence interval [CI] 1.001–1.004; p=0.002) was an independent risk factor for active BD.
Conclusion
The SII can be considered a novel predictor of BD activity.
INTRODUCTION
The clinical findings of Behçet disease (BD) and its severity are highly variable. BD is a recurrent multisystemic disease that can affect all systems, including the cardiovascular, musculoskeletal, gastrointestinal, central nervous and pulmonary systems. It can manifest with symptoms such as oral aphthous ulcers, genital ulcerations and uveitis.1,2 Disease presentation and severity vary widely among patients. Heterogeneous clinical manifestations make it difficult to determine disease activity.3 No specific test correlates with clinical findings and BD activity. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level (indicators that are known to be non-specific and can increase under various physiological and/or pathological circumstances) are the most common markers used to determine BD activity.4 Earlier studies showed that the neutrophil– lymphocyte ratio (NLR) can be used as a determiner for the increase of inflammation in patients with assorted diseases including BD.5,6
A novel inflammatory indicator, the systemic immune– inflammation index (SII), has been found to be useful for estimating the disease course in patients with various types of cancer, autoimmune diseases and coronary artery disease.7–11 The SII is cost-effective and easily calculated mathematically (platelet count × neutrophil/lymphocyte count) using peripheral blood smear. However, to the best of our knowledge, the literature includes just one study on the relationship between BD activity and SII.12 We evaluated the utility of SII in predicting disease activity in patients with BD.
METHODS
We included 513 patients aged >18 years who were previously diagnosed according to the International Study Group for BD criteria13 between June 2014 and September 2017. The medical data and laboratory findings were collected retrospectively from the hospital’s electronic data system.
Based on disease activation signs and symptoms at the time of blood collection, patients were divided into two groups, clinically active and inactive. The active group included patients with ≥1 symptoms and the inactive group included those with no symptoms for the previous 4 weeks. The following clinical data were recorded: patient demographic characteristics, initial complaint, age at the time of initial complaint, duration of illness, smoking and alcohol use, family history of BD, pathergy test results, HLA-B5 and HLA-B51 positive, systemic treatment of BD, and clinical features during the active disease period (skin and mucosal symptoms, eye, central nervous system, musculo-skeletal system, cardiovascular system, gastrointestinal system, and other organ and system involvement). In addition, complete blood count, mean platelet volume (MCV), red cell distribution width (RDW), platelet–lymphocyte ratio (PLR), SII, NLR, ESR and CRP level during both active and inactive periods were evaluated. The SII, PLR and NLR were mathematically calculated from routine complete blood cell counts. All laboratory analyses were assessed on the same day as clinical evaluation. The clinical activity in the patients was retrospectively evaluated using the Behçet Disease Current Activity Form (BDCAF) activation index from the electronic medical records data. The BDCAF is a valid and reliable tool for assessing the history and clinical features in patients with BD. The BDCAF score ranges from 0 to 12 and is calculated by adding the score of each of the scale’s indexes.14 Exclusion criteria for patients were as follows: acute infection, malignancy, pregnancy, ulcerative colitis, familial Mediterranean fever, SLE and the presence of other systemic inflammatory diseases. The study protocol was approved by the Ethics Committee (no. GO 17/789-05), the requirement for patient consent was waived by the Ethics Committee. The study was conducted as per the ethical principles described in the Declaration of Helsinki.
Statistical analysis
Statistical analysis was done with the SPSS 23.0 program for Windows (IBM Corp., Armonk, NY). Continuous variables are shown as mean (SD) and categorical variables are expressed as a percentage. The Shapiro–Wilk test was used to determine normality of data distribution. The significance of the difference between groups was determined using the independent samples t-test. Categorical variables were evaluated using Pearson chi-square test or Fisher exact test. Pearson correlation test was used to evaluate the relationship between SII and other inflammatory markers.
Binary logistic regression analysis was done to identify BD-related risk factors. Receiver operating characteristic (ROC) curve analysis was used to calculate the SII, PLR and NLR cutoff values to predict disease activity, based on Youden index.15 Statistical significance was set at a p value of 0.05.
RESULTS
We included 513 patients with BD, of which 269 (52.4%) were women and 244 (47.6%) were men. The mean (SD) age of the patients was 42.0 (12.11) years (range 18–73 years). The active group included 355 patients (69.2%; 184 men) whose mean age was 40.38 (11.72) years (range 18–70 years). The inactive group included 158 patients (30.8%; 98 women) whose mean age was 45.44 (12.29) years (range 18–73 years). The age, gender, smoking status, disease duration, and the mean SII, PLR, NLR, ESR and CRP levels differed significantly between the active and inactive groups (Table I). The mean NLR, SII, PLR, ESR and CRP levels in the patients with cardiovascular involvement were significantly higher than in patients without cardiovascular involvement (p=0.001, p<0.001, p<0.001, p<0.001 and p<0.001, respectively; Table II).
| Characteristic | All patients (%) | Active patients (%) | Inactive patients (%) | p value |
|---|---|---|---|---|
| Mean (SD) age in years | 42.00 (12.11) | 40.38 (11.72) | 45.44 (12.29) | <0.001 |
| Gender | 0.004 | |||
| Women | 269 (52.4) | 171 (48.2) | 98 (62) | – |
| Men | 244 (47.6) | 184 (51.8) | 60 (38) | – |
| Age at initial complaint (in years) | 28.0 (9.0) | 27.4 (8.7) | 29.0 (9.7) | 0.07 |
| HLA B-51 positive | 149 (29) | 118 (79.2) | 31 (20.8) | 0.37 |
| Pathergy test positive | 137 (26.7) | 108 (78.8) | 29 (21.2) | 0.57 |
| Smoking | 489 (95.3) | 339 (69.3) | 150 (30.7) | 0.04 |
| Coronary artery disease | 479 (93.3) | 333 (69.6) | 146 (30.4) | 0.92 |
| Diabetes mellitus | 486 (94.8) | 337 (69.3) | 149 (29.7) | 0.08 |
| Hypertension | 491 (95.8) | 341 (69.4) | 150 (30.6) | 0.34 |
| Hyperlipidaemia | 31 (6) | 1 9 (61.2) | 12 (38.8) | 0.08 |
| Positive family history | 8 3 (16.1) | 5 8 (69.9) | 25 (30.1) | 0.04 |
| BDCAF score | 1.0 (1.18) | 1.97 (0.91) | 0 (0) | <0.001 |
| Disease duration in years | 14.0 (9.3) | 13.0 (9.3) | 16.1 (9.0) | <0.001 |
| Haemoglobin (mg/dl) | 13.40 (1.58) | 13.28 (1.57) | 13.57 (1.59) | 0.51 |
| White cell count (cmm) | 7.60 (3.08) | 8.73 (3.36) | 7.08 (1.92) | <0.001 |
| Neutrophil count (cmm) | 4.80 (2.73) | 5.84 (2.99) | 4.09 (1.44 | <0.001 |
| Lymphocyte count (cmm) | 2.00 (1.19) | 2.11 (1.35) | 2.28 (0.70 | 0.14 |
| Platelet count (cmm) | 260.0 (94.9) | 282.2 (102.2) | 250.6 (71.7) | <0.001 |
| MCV | 84.80 (6.31) | 83.47 (6.10) | 85.02 (6.64) | 0.01 |
| RDW (%) | 14.30 (1.88) | 14.79 (1.89) | 14.65 (1.85) | 0.43 |
| NLR | 2.29 (2.94) | 3.50 (3.39) | 1.88 (0.75) | <0.001 |
| ESR (mm in first hour) | 11.00 (18.05) | 19.54 (20.25) | 11.15 (9.93) | <0.001 |
| C-reactive protein (mg/L) | 0.54 (4.11) | 2.35 (4.82) | 0.57 (0.82) | <0.001 |
| SII | 790.7 (751.6) | 932.7 (852.9) | 471.7 (232.2) | <0.001 |
| PLR | 143.53 (81.1) | 154.86 (90.5) | 118.06 (45.2) | <0.001 |
| Medications used | ||||
| Colchicine | 332 (64.7) | 218 (65.7) | 114 (34.3) | 0.019 |
| Systemic corticosteroid | 7 3 (14.2) | 6 0 (82.2) | 13 (17.8) | 0.009 |
| Azathioprine | 82 (16) | 5 8 (70.7) | 24 (29.3) | 0.74 |
| Cyclosporine | 7 (1.4) | 5 (71.4) | 2 (28.6) | 0.89 |
| Acetylsalicylic acid | 134 (26.1) | 8 2 (61.2) | 52 (38.8) | 0.02 |
| Warfarin | 32 (6.2) | 1 7 (53.1) | 15 (46.9) | 0.04 |
| Heparin | 11 (2.2) | 8 (72.7) | 3 (27.3) | 0.79 |
| NSAIDs | 22 (4.3) | 1 9 (86.4) | 3 (13.6) | 0.07 |
| Interferon-alpha | 40 (7.8) | 28 (70) | 12 (30) | 0.90 |
| Penicillin | 9 0 (17.5) | 5 1 (56.7) | 39 (43.3) | 0.005 |
| Anti-TNF agents | 18 (3.3) | 9 (50) | 9 (50) | 0.07 |
| Methotrexate | 4 (0.7) | 4 (100) | 0 (0) | 0.18 |
BDCAF Behçet Disease Current Activity Form MCV mean platelet volume RDW red cell distribution width NLR neutrophil– lymphocyte ratio ESR erythrocyte sedimentation rate SII systemic immune–inflammation index PLR platelet–lymphocyte ratio NSAIDs non-steroidal anti-inflammatory drugs TNF tumour necrosis factor
| Organ/lesion | (n) | NLR | ESR (mm in first hour) | CRP (mg/L) | SII | PLR |
|---|---|---|---|---|---|---|
| Oral aphthae | Present (115) | 2.87 (1.82) | 16.58 (16.54) | 1.69 (2.70) | 781.2 (441.4) | 143.29 (54.1) |
| Absent (398) | 3.04 (3.20) | 16.95 (18.47) | 1.83 (4.44) | 793.5 (820.1) | 143.60 (87.4) | |
| p value | 0.49 | 0.84 | 0.74 | 0.83 | 0.96 | |
| Genital ulcer | Present (40) | 3.46 (2.38) | 24.34 (23.83) | 3.14 (5.10) | 1173.3 (1383.5) | 159.40 (66.6) |
| Absent (473) | 2.96 (2.98) | 16.25 (17.37) | 1.69 (4.00) | 758.4 (664.2) | 142.18 (82.1) | |
| p value | 0.30 | 0.047 | 0.09 | 0.06 | 0.19 | |
| Erythema nodosum- | Present (31) | 2.84 (1.12) | 26.71 (23.39) | 2.44 (2.62) | 835.9 (498.3) | 141.52 (79.02) |
| like lesions | Absent (482) | 3.01 (3.02 | 16.28 (17.53) | 1.76 (4.19) | 787.8 (765.3) | 143.65 (81.3) |
| p value | 0.75 | 0.028 | 0.37 | 0.73 | 0.88 | |
| P P L | Present (28) | 2.92 (1.14) | 14.65 (14.28) | 1.91 (2.54) | 818.3 (407.4) | 134.44 (42.0) |
| Absent (485) | 3.00 (3.02) | 16.99 (18.24) | 1.79 (4.19) | 789.1 (767.0) | 144.05 (82.8) | |
| p value | 0.87 | 0.52 | 0.88 | 0.84 | 0.54 | |
| Ocular involvement | Present (51) | 3.98 (4.31) | 17.74 (19.21) | 1.37 (2.35) | 982.1 (819.3) | 133.74 (73.1) |
| Absent (462) | 2.89 (2.74) | 16.78 (17.94) | 1.84 (4.26) | 769.6 (741.7) | 144.61 (82.0) | |
| p value | 0.08 | 0.72 | 0.44 | 0.08 | 0.36 | |
| Uveitis | Present (39) | 3.72 (4.72) | 18.37 (19.28) | 1.07 (1.82) | 887.9 (794.8) | 129.18 (58.3) |
| Absent (474) | 2.94 (2.75) | 16.74 (17.96) | 1.86 (4.24) | 782.7 (748.3) | 144.71 (82.6) | |
| p value | 0.31 | 0.59 | 0.03 | 0.40 | 0.25 | |
| Arthritis | Present (40) | 3.33 (3.57) | 19.73 (16.40) | 1.93 (1.98) | 812.2 (500.5) | 147.29 (59.2) |
| Absent (473) | 2.97 (2.89) | 16.62 (18.18) | 1.79 (4.24) | 788.9 (769.5) | 143.21 (82.7) | |
| p value | 0.45 | 0.49 | 0.83 | 0.85 | 0.76 | |
| Arthralgia | Present (105) | 2.88 (2.84) | 16.17 (15.88) | 1.31 (2.25) | 686.6 (408.5) | 139.33 (52.7) |
| Absent (408) | 3.03 (2.97) | 17.05 (18.59) | 1.93 (4.47) | 817.5 (815.2) | 144.61 (87.0) | |
| p value | 0.63 | 0.65 | 0.050 | 0.02 | 0.43 | |
| GIS involvement | Present (12) | 3.85 (2.56) | 27.33 (30.92) | 8.40 (13.25) | 1548.7 (2284.6) | 166.09 (78.4) |
| Absent (501) | 2.98 (2.95) | 16.61 (17.59) | 1.64 (3.50) | 772.6 (670.5) | 142.99 (81.2) | |
| p value | 0.31 | 0.25 | 0.10 | 0.26 | 0.33 | |
| Cardiovascular | Present (60) | 4.78 (4.27) | 34.49 (28.28) | 6.17 (8.78) | 1401.4 (1053.9) | 231.00 (161.3) |
| involvement | Absent (453) | 2.76 (2.64) | 14.67 (14.99) | 1.22 (2.47) | 709.8 (662.5) | 131.94 (53.9) |
| p value | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| CNS involvement | Present (26) | 5.82 (5.19) | 24.83 (22.30) | 2.85 (5.06) | 1573.9 (1195.5) | 187.03 (68.0) |
| Absent (487) | 2.85 (2.70) | 16.46 (17.74) | 1.75 (4.06) | 748.9 (697.9) | 141.20 (81.2) | |
| p value | 0.008 | 0.083 | 0.21 | 0.002 | 0.005 |
Data are presented as mean (SD) p<0.05 is statistically significant NLR neutrophil–lymphocyte ratio ESR erythrocyte sedimentation rate SII systemic immune–inflammation index PLR platelet–lymphocyte ratio CRP C-reactive protein PPL papulopustular-like lesions GIS gastrointestinal system CNS central nervous system
Colchicine, systemic corticosteroid, acetylsalicylic acid, warfarin and penicillin use was significantly higher in the active than in the inactive BD group (p=0.019, p=0.009, p=0.02, p=0.04 and p=0.005, respectively); however, there was no significant difference in the use of other drugs (Table I).
We found that the SII was correlated positively with the NLR, PLR, ESR and CRP level (coefficient values [95%] were r=0.672, r=0.711, r=0.422 and r=0.427, respectively; p<0.001 for all). Multivariate logistic regression analysis showed that age (OR 0.991; 95% confidence interval [CI] 0.967–1.015; p=0.45), gender (OR 0.712; 95% CI 0.451–1.122; p=0.14), disease duration (OR 0.989; 95% CI 0.959–1.019; p=0.47), SII (OR 1.003; 95% CI 1.001–1.004; p=0.002), NLR (OR 1.414; 95% CI 0.969–2.063; p=0.07), and PLR (OR 0.997; 95% CI 0.990–1.003; p=0.33) were associated with BD activation. However, it also showed that only SII was independently associated with the prediction of BD activation. ROC indicated that area under the curve (AUC) values for SII, NLR and PLR were 0.758, 0.750 and 0.656, respectively (p<0.001 for all; Fig. 1). The SII cut-off value was 526.23 (70.4% sensitivity and 70.3% specificity). Moreover, the NLR and PLR cut-off values were 2.11 (67.3% sensitivity and 68.4% specificity) and 119.63 (63.4% sensitivity and 63.3% specificity), respectively (Fig. 1).
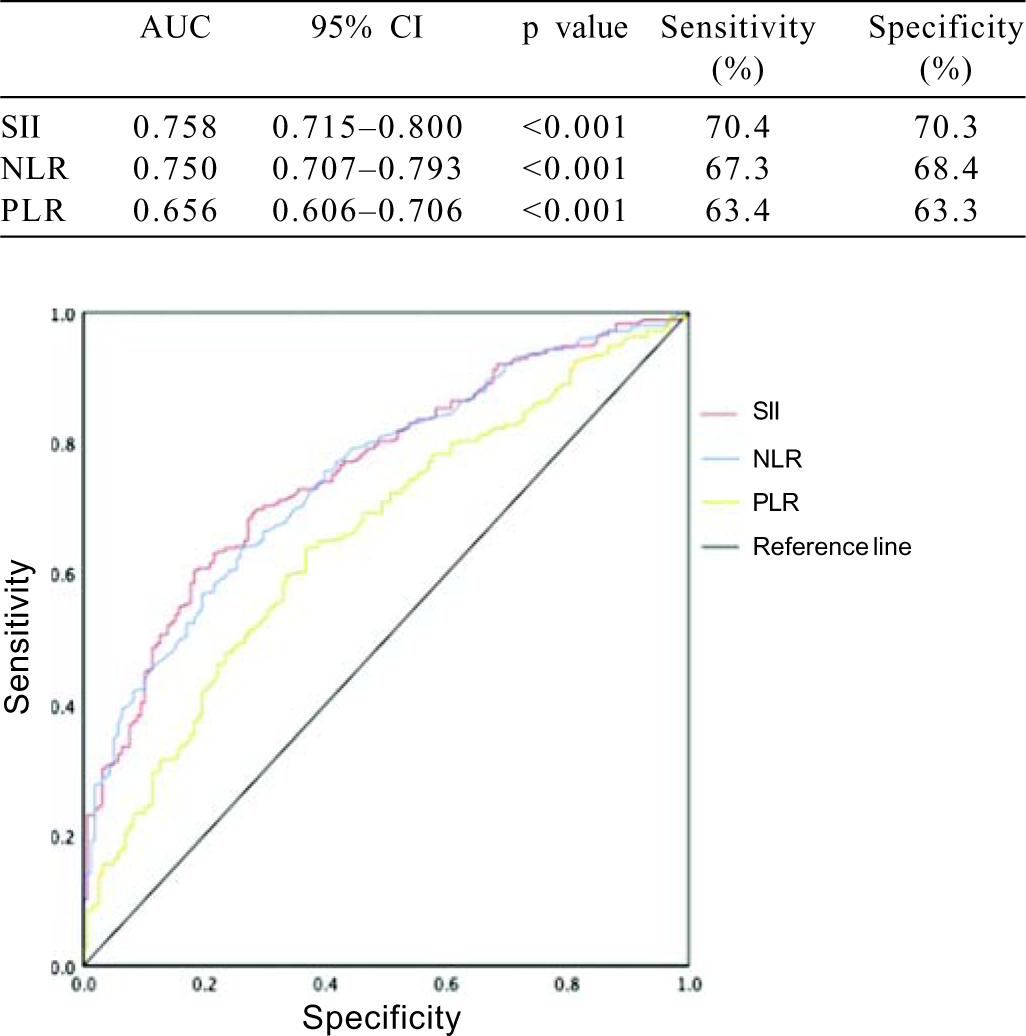
- Receiver operating characteristic curves for the systemic immune–inflammation index (SII), neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR)
DISCUSSION
There is no specific laboratory test for BD activity that correlates with clinical findings. In our study, the SII, NLR, PLR, ESR and CRP levels were significantly higher in the active BD group than in the inactive BD group.
Earlier studies have shown that well-known biomarkers of inflammation, such as the NLR, PLR, ESR and CRP levels can be used to determine BD activation.16,17 Serum levels of beta 2-microglobulin and amyloid A protein were found to be significantly higher in patients with active BD than in those with inactive BD; however, routine testing of these markers is expensive and not yet standardized. Furthermore, there is insufficient evidence to recommend the use of these markers in routine practice.18,19
The role of neutrophils in the pathogenesis of BD was first understood in the literature in 1975, demonstrating increased polymorphonuclear leukocyte activity. After that, colchicine started to be used in patients with BD because it inhibits neutrophil chemotaxis.20 Research on neutrophil-related markers such as NLR in evaluating BD activity has increased over time. The NLR was first developed as a stress-related index in patients with systemic inflammatory diseases. It has been used to predict survival and prognosis in patients with solid tumours and critical diseases such as coronary artery disease and acute pancreatitis.21–23
In a meta-analysis of 14 studies in 2018, NLR was evaluated in patients with active BD compared to inactive and/or healthy control groups. It has been suggested that NLR can be used as a practical, inexpensive and simple marker, even though BD is a heterogeneous disease with personal distinctions.24 We too found that NLR was significantly higher in active than in patients with inactive BD.
It was reported that SII (calculated using the complete blood count) can be used as a prognostic indicator in various cancers.25–27 Hu et al.26 developed SII in 2014 using calculations according to the parameters of a routine blood examination consisting of lymphocyte, neutrophil and platelet counts, and suggested that SII is a strong prognostic indicator following curative resection for hepatocellular carcinoma.
To the best of our knowledge, the literature has just one study correlating SII and BD. Tanacan et al.12 in 2021 suggested that SII could be used to determine BD activity and recommend that the cut-off value for SII is >552 (81% sensitivity and 82% specificity) consistent with this recent study. ROC analysis in our study showed that SII was significantly higher in the active than in the inactive BD group. The cut-off value for SII in the present study was lower (526.23×103 mm-3 [70.4% sensitivity, 70.3% specificity]) than that reported by Tanacan et al.,12 which might have been due to the smaller patient population in their study (513). In our study, patients with central nervous system and cardiovascular system involvement had a significantly higher SII (p=0.002 and p<0.001, respectively) than those without, whereas there was no significant difference in the SII according to other organ involvement.
Öztürk et al.28 reported that the NLR was significantly higher (p<0.001) in patients with active BD (n=65) than in those with inactive BD (n=62), and noted a moderate positive correlation between the CRP level and NLR in patients with BD. ROC curve analysis showed that in their patients with BD, the optimum cutoff value for NLR is 1.29 (97% sensitivity and 77% specificity; AUC=0.691; 95% Cl 0.600–0.782; p<0.001). Similarly, our study found that the NLR was significantly higher in patients with active BD than in those with inactive BD (p<0.001). Additionally, the cut-off value for the NLR was 2.11 (sensitivity 67.3% and specificity 68.4%; AUC=0.75) in our study. The NLR cut-off value was found to be higher than in this study.
A study investigated the role of NLR, ESR and CRP levels in the activity of BD and susceptibility to thrombosis and reported a correlation between ESR and thrombosis. ESR was significantly higher in the active BD group with thrombosis compared to the active BD group without thrombosis; however, there was no significant difference in the NLR or CRP levels between the patients with active BD with and without thrombosis.29 Another study involving 60 patients with BD showed that the ESR and CRP levels were significantly higher in patients with BD with thrombosis (n=22) than in those without thrombosis (n=38; p<0.001 and p<0.001, respectively).30 In this study, patients with BD with vascular involvement such as venous thrombosis, pulmonary embolism, Budd–Chiari syndrome or thrombosis of the major veins were grouped under patients with cardiovascular involvement. The diagnosis of cardiovascular involvement was confirmed by clinical features, doppler ultrasonography, multislice helical CT and/or angiography. In addition, the NLR, PLR, SII, ESR and CRP levels were significantly higher in patients with BD and cardiovascular involvement (n=60) than in those without cardiovascular involvement (p=0.001, p<0.001, p<0.001, p<0.001 and p<0.001, respectively). To the best of our knowledge, our study is the largest to investigate peripheral inflammatory markers in patients with BD with cardiovascular involvement. The primary limitation of our study is its single-centre retrospective design. Hence, larger, prospective and multicentre studies are required to support our findings.
Conclusion
This large retrospective study shows that SII is an inexpensive and beneficial evaluation tool for assessing BD activation. Clinicians should be alert to the possibility of BD activation in patients with an SII >526.23.
ACKNOWLEDGEMENTS
This article is based on Dr Dilek Mentesoglu’s completed dermatology specialization thesis, titled ‘The investigation of neutrophil/lymphocyte ratio, sedimentation, and C-reactive protein levels according to target organ and tissue involvement in patients with Behçet disease’ supervised by Professor Dr Nilgün Atakan (Thesis in Dermatology and Venereology, Hacettepe University, Faculty of Medicine, Ankara, Turkey, 2018).
References
- Behçet's disease-a contemporary review. J Autoimmun. 2009;32:178-88.
- [CrossRef] [PubMed] [Google Scholar]
- Behçet syndrome: A contemporary view. Nat Rev Rheumatol. 2018;14:107-19.
- [CrossRef] [PubMed] [Google Scholar]
- Behçet's disease: Review with emphasis on dermatological aspects. An Bras Dermatol. 2017;92:452-64.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a relation between clinical disease activity and acute phase response in Behcet's disease? Int J Dermatol. 2014;53:250-4.
- [CrossRef] [PubMed] [Google Scholar]
- The neutrophil-lymphocyte ratio and Behcet disease. Angiology. 2016;67:297.
- [CrossRef] [PubMed] [Google Scholar]
- The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb. 2016;22:405-11.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index could estimate the cross-sectional high activity and the poor outcomes in immunosuppressive drug-naïve patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology. 2019;24:711-17.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. 2020;24:610-18.
- [CrossRef] [PubMed] [Google Scholar]
- The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146:692-8.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50:e13230.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index combined with ferritin can serve as a reliable assessment score for adult-onset Still's disease. Clin Rheumatol. 2021;40:661-8.
- [CrossRef] [PubMed] [Google Scholar]
- A cutoff value for the systemic immune-inflammation index in determining activity of Behçet disease. Clin Exp Dermatol. 2021;46:286-91.
- [CrossRef] [PubMed] [Google Scholar]
- Behcet's disease: Evaluation of a new instrument to measure clinical activity. Rheumatology. 1999;38:728-33.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of the Youden index and its associated cutoff point. Biometrical J. 2005;47:458-72.
- [CrossRef] [PubMed] [Google Scholar]
- Serum PLR and LMR in Behçet's disease: Can they show the disease activity? Medicine (Baltimore). 2017;96:e6981.
- [CrossRef] [PubMed] [Google Scholar]
- The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behçet's syndrome. Kaohsiung J Med Sci. 2015;31:626-31.
- [CrossRef] [PubMed] [Google Scholar]
- Serum beta 2-microglobulin reflects disease activity in Behçet's disease. Rheumatol Int. 2002;22:5-8.
- [CrossRef] [PubMed] [Google Scholar]
- Serum amyloid-A in Behçet's disease. Clin Rheumatol. 2014;33:1165-7.
- [CrossRef] [PubMed] [Google Scholar]
- Leucocyte movement and colchicine treatment in Behcet's disease. Lancet. 1975;2:813.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of neutrophil to lymphocyte ratio in outcomes of patients with acute coronary syndrome. Arch Med Res. 2010;41:618-22.
- [CrossRef] [PubMed] [Google Scholar]
- A novel predictive model based on preoperative blood neutrophil-to-lymphocyte ratio for survival prognosis in patients with gastric neuroendocrine neoplasms. Oncotarget. 2016;7:42045-58.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-52.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio, mean platelet volume and platelet-to-lymphocyte ratio in Behçet's disease and their correlation with disease activity: A meta-analysis. Int J Rheum Dis. 2018;21:2180-7.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297-304.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212-22.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7:44185-93.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophillymphocyte ratio and carotid-intima media thickness in patients with Behçet disease without cardiovascular involvement. Angiology. 2015;66:291-6.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil/lymphocyte ratio and mean platelet volume in Behçet's disease. Eur Rev Med Pharmacol Sci. 2016;20:3045-50.
- [Google Scholar]
- Increased mean platelet volume in Behcet's disease with thrombotic tendency. Tohoku J Exp Med. 2010;221:119-23.
- [CrossRef] [PubMed] [Google Scholar]




