Translate this page into:
Active case-finding for tuberculosis in India
Corresponding Author:
Mohan Natrajan
ICMR-National Institute for Research in Tuberculosis, No. 1, Mayor Sathiyamoorthy Road, Chetpet, Chennai 600031, Tamil Nadu
India
mohan.n@nirt.res.in
| How to cite this article: Prathiksha G, Daniel BD, Natrajan M. Active case-finding for tuberculosis in India. Natl Med J India 2019;32:90-95 |
Abstract
Early identification of presumptive tuberculosis (TB) cases through active case-finding (ACF) would be an important complementary strategy to meet the national urgency in accelerating case detection to achieve the goals of 'End TB' strategy. ACF activities have yielded additional cases in different vulnerable groups in India. The yield of cases depends on the screening tool available, the characteristics of the high-risk population being screened, and most importantly, the linkage between effective diagnostic and treatment facilities. The ACF strategy could be both economically and epidemiologically relevant if it could bring down the level of transmission. This needs long-term research focusing on outcomes such as cases averted and reduction in the prevalence of the disease. Available evidence suggests that ACF is likely to be feasible in Indian settings but needs to be scaled up rapidly to create a good impact.Introduction
Globally, India accounts for one-fourth of the burden of tuberculosis (TB). India is among the six leading countries that contribute to 60% of new cases globally. [1] The ′End TB′ Strategy calls for 80% reduction in incidence and 90% reduction in mortality of TB by 2030 compared with 2015. [2] Control of TB is currently dependent on three main strategies: (i) case-finding and treatment of TB; (ii) treatment of latent TB infection; and (iii) vaccination. Treatment of latent TB infection is not being widely done and vaccination has minimal impact on prevention of TB in adults. These two strategies have little impact on the incidence of TB in India. This leaves case-finding and treatment of TB as the principal means of control of transmission and reduction in the incidence of TB. [3],[4] Passive case-finding (PCF) has been done over the years, where those with symptoms seek healthcare facilities. This method has its disadvantages as there may be delays both from the patient and the health system. Such delays may miss the diagnosis or result in delayed diagnosis, leading to increased risk of suffering, death and catastrophic economic consequences to the patient. These missed opportunities play a crucial role in sustaining transmission as it leads to longer periods of transmission, especially in populations with poor living and working conditions. [5]
A large pool of undetected cases exists in many high-risk and vulnerable groups. Barriers such as lack of awareness, lack of regular healthcare services and competing priorities for time and money make the healthcare facilities inaccessible to them. Strategies such as active case-finding (ACF), which address this issue of inaccessibility play a vital role in scaling up case detection rates in India. [2]
Project Axshya done in 300 districts among high-risk groups across India showed that ACF was able to efficiently detect a large number of presumptive and active TB cases by using community volunteers with proper training and supervision. [6]
The options available for active screening include symptom screening and chest X-ray. Cough for more than 2 weeks when used as a primary screening tool has a sensitivity of 56.2% and a specificity of 95.3%. [3] Chest X-ray as an initial screening tool has a sensitivity of 76.6% and a specificity of 97%. [3] Extensive literature reviews have found that ACF can reduce the burden of TB in terms of disease prevalence, incidence, mortality and morbidity. [5],[7]
WHO recommends active screening among high-risk groups such as healthcare workers (HCWs), urban slum dwellers, contacts of patients with TB, prison inmates, miners, people with diabetes, smokers and HIV-positive individuals.5 Chest X-ray among those who have cough for >2 weeks has a sensitivity of 66.8% and a specificity of 87.8%.3 The choice of screening tool used for ACF varies across different vulnerable groups or settings and is also based on the available resources [Figure - 1]. [5],[7] We focus on the various ACF activities that have taken place across selected high- risk groups, the need for such ACF activities in these groups, and the benefits and challenges of ACF in India.
 |
| Fidure 1: Framework for active case-finding among high-risk groups in India |
Active Case-Finding Activities in India
Despite the increase in notification due to NIKSHAY, there is still a large undetected burden of patients with TB. All patients with chest symptoms especially those who have poor access to healthcare facilities do not seek healthcare. [6] In this scenario, ACF for TB remains an important integral part of TB control in high-risk groups. [Table - 1] shows the various ACF activities done in selected high-risk groups and their yield.
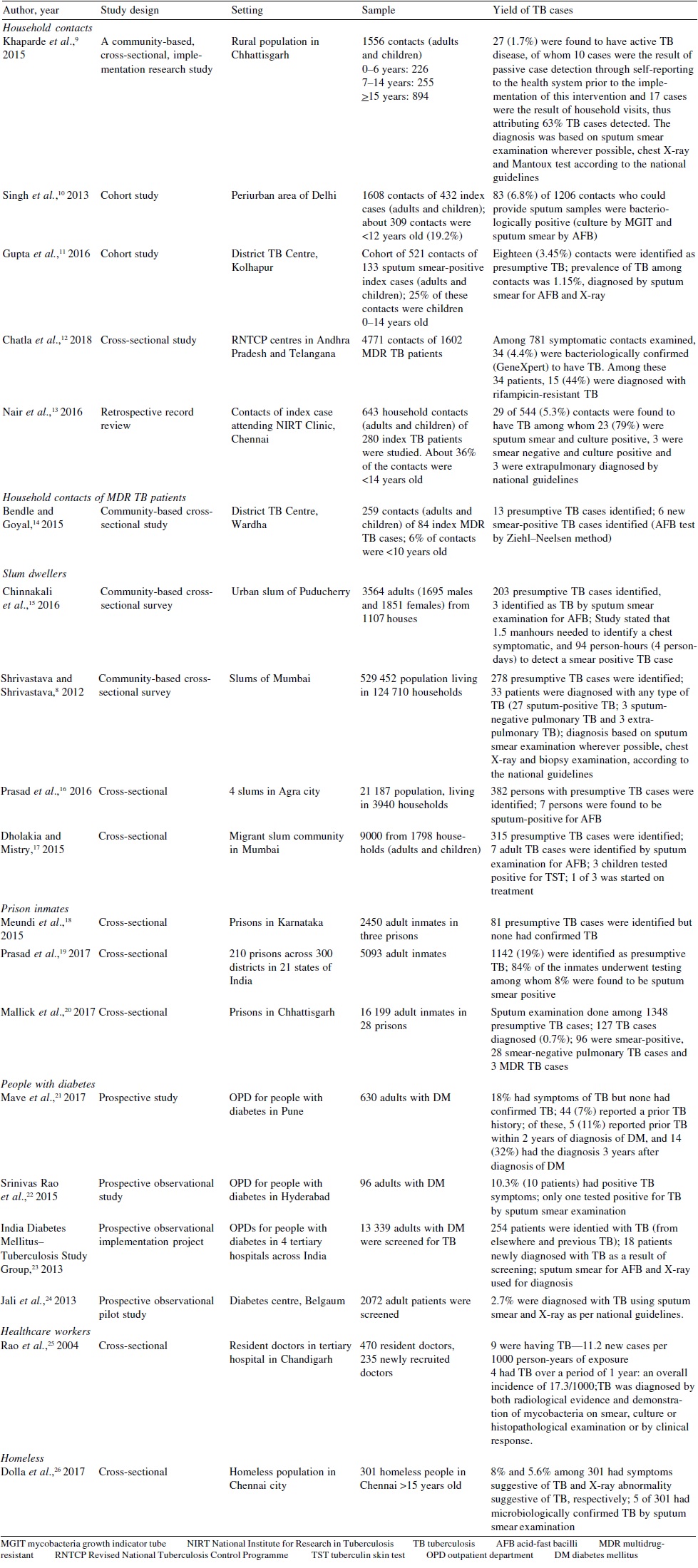
Urban slums
A study conducted using ACF strategy in Mumbai reported that 278 TB suspects were identified among 529 452 people. Among them, 33 patients were diagnosed with any type of TB8 [Table - 1]. An ACF study done among slum-dwellers of Puducherry reporte that 1.5 person-hours were needed to identify a chest symptomatic and 94 person-hours (4 person-days) to detect a case of smear- positive TB [Table - 1]. [15] Prasad et al. in a study done among slum- dwellers of Agra highlighted that all household members have to be screened for symptoms of TB to get a high yield of presumptive cases. In addition, mere referral for sputum examination was not enough and people need to be supported to reach the healthcare facility for sputum examination. They reported that only 40% of presumptive cases screened by ACF reached the health facility for testing on their own [Table - 1].[16]
Healthcare workers (HCWs)
Limited studies are available regarding ACF for TB among HCWs. A study conducted in Chandigarh provides information about ACF for TB among HCWs. The study showed that among 470 resident doctors who were interviewed, 9 were suffering from TB, which is 11.2 new cases per 1000 person-years of exposure. Of these 9 doctors, 6 had extrapulmonary disease. Among 235 newly recruited doctors, 4 had TB over a period of 1 year, which is an overall incidence of 17.3/1000 person-years. An important finding was that extrapulmonary disease was more common than pulmonary TB among them [Table - 1].[25]
Prison inmates
Project Axshya, which was implemented in 300 districts across 21 states, studied 210 prisons in India. In this study, 5093 inmates were screened for TB, of which 1142 (19%) were identified as presumptive TB cases. Nearly 84% of the inmates underwent testing, among whom 8% were found to be sputum smear- positive. This ACF yielded an additional 80 TB patients who otherwise would have been missed in the existing system. [19] An ACF study done in Karnataka reported that among 2450 inmates in three prisons, there were 81 presumptive TB suspects and 10 were already on DOTS (directly observed treatment, short course). [18] Mallick et al. had reported that ACF activities conducted among 28 prisons in Chhattisgarh led to an increase of 39% and 38% in presumptive TB examination rate and TB case notification rate, respectively, among prison inmates [Table - 1]. [20]
Homeless population
Studies estimating the burden of TB among the homeless in India are limited. A study done by Dolla et al. in Chennai city found the prevalence of TB among homeless to be 5/301 (1661/100 000 population) using ACF [Table - 1]. [26]
People with diabetes
In a study done in Pune, no new TB cases were detected among patients with diabetes mellitus (DM), even when two-thirds of the DM patients had poor glycaemic control. [21] In another study done in Hyderabad, the authors reported that of 96 patients with DM screened, 10.3% (10 patients) had symptoms suggestive of TB. Of these, only 1 tested positive for TB. [22] Another study to ascertain the feasibility, outcomes and challenges for screening TB among people with DM was done across six DM clinics situated in tertiary hospitals in India. Over the screening period which occurred over three-quarters of the year, about 26% of 7218, 52% of 12 237, and 48% of 11 691 patients with DM were screened. A total of 254 patients were identified with TB, among whom 18 patients were newly diagnosed with TB as a result of screening and the remaining were patients already diagnosed elsewhere [Table - 1]. [23]
Contacts
A study done in rural districts of Chhattisgarh reported that on an average, for every 11 households where screening for contacts was done, there was one case of active TB. They attributed 63% of additional cases to ACF. They also reported that 1 in 4 of the symptomatic contacts did not report to the Revised National TB Control Programme for sputum examination despite repeated counselling. [9] A study done in peri-urban areas of Delhi by Singh et al. concluded that 6.8% of the household contacts of patients with TB were diagnosed as active TB by the ACF strategy over a period of 4 years including 2 years of follow-up. The study showed that 84.6% of the prevalent cases and 70.9% of the incident cases were smear-negative and could be diagnosed by culture [Table - 1]. [10]
Need For Screening For TB In Selected High- Risk Groups
Slums
In many developing countries including India, rapid urbanization amidst lack of proper urban planning has resulted in increasing number of slums and informal settlements in many cities. Rates of transmission of TB are generally more in slums than in non-slum areas. [27],[28] Secondary data analysis from the National Family Health Survey-3 showed that slum-dwellers were at a risk of having TB 2.4-times more than non-slum-dwellers. [29] Though the yield of ACF is on an average of <1% of TB cases [Table - 1], it is still a significant number taking into consideration the huge slum population in India. Similar results were reported from a study done in the slums in Bangladesh. [30] ACF for TB done in African countries-Uganda, Ethiopia and South Africa- had high yields ranging from 4% to 13%. [31],[32],[33] This could be because of the extreme burden of TB in such countries due to HIV transmission.
There is also evidence from Indian settings that despite repeated counselling sessions to undergo sputum examination among the high-risk population, a significant proportion do not undergo the examination at the field level. A study among slum-dwellers of Mumbai reported that 51.8% of the TB suspects did not go for sputum examination because their job timings clashed with the timings of the designated microscopy centres (DMC).8 These factors need to be considered to design effective interventions such as using field-level diagnostics so that presumptive cases are not missed.
Healthcare workers
The annual risk of TB infection in India among HCW is 5%, which is more than three times the national average of 1.5%. [34],[35] The incidence among resident doctors-11.2 new TB cases per 1000 person-years of exposure-is almost 10-times higher than the general population. [25] The probability of nosocomial transmission is expected to be high in India due to its large burden of TB. [36] In a prevalence study among 726 HCWs, 50% were found to have latent TB infection. [37] All these data suggest a high burden for TB in HCWs. A systematic review by Baussano et al. estimated latent TB infection among HCWs in high TB burden countries to be more than 100 cases per 100 000 persons. [38] The expected probability of HCW acquiring TB is large is India, with its high burden of TB and a large load of ′open cases′ visiting hospitals. The transmission of TB in healthcare settings can be taken as a proxy measure for the overall infection control practices in the healthcare system. [39] Infection control measures, especially at the primary care level, are inadequate in India. [36] A retrospective study by Gopinath et al. estimated the annual incidence of pulmonary TB and extrapulmonary TB to be 0.35-1.80 and 0.34-1.57 per 1000 HCWs, respectively. [40] HCWs constitute a very high-risk group for TB, and unfortunately active screening for TB among HCWs is scarce in India. ACF provides a valuable opportunity to identify TB cases in one of the most vulnerable populations for TB infection in India.
Prison inmates
Overcrowding is common in Indian prisons. [20] The National Crime Records Bureau of India reported a total of 419 623 inmates as on December 2015. [41] TB is 23-times higher in prison inmates than in the general population. TB is also the most common cause of death among prisoners in developing countries. [19] ACF can identify a large number of presumptive TB cases-as high as 19%. [19] Ali et al. in Ethiopia did ACF and identified only 4.9% of TB suspects. [42]
Though prisons seem to be a ′closed′ system, they have a high potential for transmission of TB in the community through staff in the prison, visitors, prison transfers, recidivists and also they are most likely to enter, leave and re-enter at a later date. [43]
Meundi et al. in their study of three Indian prisons, more than two-thirds of presumptive TB cases who consulted the prison medical officer did not find any relief for their symptoms. [18] This reveals the unmet need for medical care among prisoners. Similar findings were reported by Vinkeles Melchers et al. in a systematic review of TB screening in prisons, which stated that about 21% of all studies described the lack of a well-organized health system for prisoners. [44] All these facts support the identification and control of TB in prisons on priority.
Homeless population
The 2011 census reports that 26% of the homeless population in India is contributed by the five metropolitan cities-Delhi, Kolkata, Mumbai, Chennai and Bengaluru. TB estimates are 20-times higher in the homeless than the general population in many industrialized countries. [45] Studies in the USA found a high burden of TB in the homeless population. [46] A 5-year study based on autopsy findings done at Lady Hardinge Medical College, New Delhi, among homeless unclaimed people, revealed that 27% deaths were due to pulmonary TB. [47] Homeless populations are particularly recalcitrant to traditional TB control activities due to challenges with contact tracing, poor access to healthcare and lack of longitudinal follow- up. Evidence-based screening and treatment strategies should be developed to improve health outcomes in this population.
People with diabetes
DM increases the risk of developing active TB by two to three times. Patients with TB have delayed culture conversion and have higher risk of recurrence after treatment completion.22, 48, 49 While it is feasible to screen TB among people with DM, the yield of active cases is very little. Jeon et al. reported that screening among patients with DM yielded active TB prevalence rates ranging from 1.7% in Sweden to 36% in Korea. [50] Nasa et al. identified 11 cases of TB by screening 213 patients with DM in the Republic of Marshal Islands. [51] Taking into consideration the low yield and large number of people with diabetes in India, there is a need to identify specific risk factors among them so that active screening could be done effectively. [21]
Contacts
Low-income countries with a high burden of TB do not have well- established contact screening programmes due to limited resources. [52] Even in countries with contact screening as a routine part of the programme, there are many challenges of implementation. [53],[54] About 2.3% of pulmonary TB patients are detected by screening of close contacts in low- and middle- income countries. [55] Contact screening provides a high yield of active TB cases-6.8%. The yield for active TB cases in China ranges from 0% to 6.9%. China has a similar burden of TB as India. [56],[57],[58]
Benefits of ACF
The impact of ACF on the incidence of TB has been proposed by various mathematical models. Murray and Salomon concluded that in combination with DOTS, ACF would yield enormous benefits in areas with a high burden of TB.[59] India has a high prevalence of TB and efforts which include ACF combined with the DOTS programme could avert millions of cases and deaths due to TB. The ACF strategy could be both economically as well as epidemiologically relevant if it could bring down the level of transmission of TB. The impact may not be evident in short-term research studies but long-term studies may be able to capture it. Modelling done by Azman et al. for ACF in three countries namely China, India and South Africa has reported that ACF for a period of 2 years would increase the yield of cases to 25% in the first year for diagnosis and treatment. [58] It reduced the average duration of untreated disease from 20 months to 17.3 months in India. This period of untreated disease is important as it is the high-risk period for transmission. [58] It could further avert 277 deaths among 1 million people over a period of 10 years. By early detection and management of undiagnosed TB through ACF, disease transmission will reduce and eventually bring down the disease burden when implemented effectively with other strategies. Also, every case averted represents a potential saving of cost of treatment for the health system. [6]
Challenges For ACF
The cost and volume of activities involved in ACF are immense. Murray and Salomon have shown that this activity would be enormously useful in settings which have a lower case detection, high prevalence and moderate to high treatment completion.59 The study concluded that interventions for only 2 years, not considering future effects, would underestimate medium-term impact over a period of 10 years by 85% in India. [58] There is a possibility to have high false-positive results during screening especially when the diagnostic test used has a suboptimal specificity. The proportion of false-positive TB cases is usually inversely proportional to the prevalence of TB. [4] ACF strategy screens high-risk groups at certain intervals. There are people who might become infectious between the screening periods and delay their health-seeking as they rely on regular screening. ACF can be effective only if PCF is strengthened through providing accessible diagnostic facilities and promoting positive health-seeking behaviour of the vulnerable population. [11]
Conclusion
Depending on PCF alone is insufficient to meet the ambitious TB elimination targets in India. Strengthening case detection is essential and a dependable strategy to combat the burden of TB in India till we find breakthrough disease prevention measures. With the available evidence, the ACF strategy is likely to be feasible in our settings but needs to be scaled up rapidly for a better impact. However, the cost of implementing ACF will be challenging. Ascertaining the effectiveness of ACF strategy on the long run depends on outcomes such as cases averted and reduction in prevalence and not on the number of cases found by ACF. Future studies must focus on generating evidence in these aspects, which will give more insight about ACF and its use in India.
Conflicts of interest. None declared
| 1. | World Health Organization. Global Tuberculosis Report 2016. Available at www.apps. who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf (accessed on 20 Jul 2017). [Google Scholar] |
| 2. | World Health Organization. Implementing the End TB Strategy: The Essentials. 2015. Available at www.who.Int/tb/publications/2015/end_tb_essential.pdf (accessed 20 Jul 2017). [Google Scholar] |
| 3. | Central TB Division. National Strategic Plan for Tuberculosis Elimination. 2017. Available at www.tbcindia.gov.in/writereaddata/NSP%20Draft%2020.02.2017% 201.pdf (accessed on 21 Jul 2017). [Google Scholar] |
| 4. | Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: Historical perspective and future prospects. Int J Tuberc Lung Dis 2005; 9: 1183-203. [Google Scholar] |
| 5. | World Health Organization. Systematic screening for active tuberculosis: Principles and recommendations. Available at www.apps.who.int/iris/bitstream/10665/84971/ 1/9789241548601_eng.pdf?ua=1 (accessed on 20 Jul 2017). [Google Scholar] |
| 6. | Prasad BM, Satyanarayana S, Chadha SS, Das A, Thapa B, Mohanty S, et al. Experience of active tuberculosis case finding in nearly 5 million households in India. Public Health Action 2016; 6: 15-18. [Google Scholar] |
| 7. | Central TB Division. Technical and operational guidelines for TB control in India. 2016. Available at www.tbcindia.nic.in/index1.php?lang=1&level=2&sublinkid= 4573&lid=3177 (accessed on 5 Jun 2017). [Google Scholar] |
| 8. | Shrivastava SR, Shrivastava PS. Tuberculosis: Active case finding survey in an urban area of India. J Res Health Sci 2012; 13: 19-23. [Google Scholar] |
| 9. | Khaparde K, Jethani P, Dewan PK, Nair SA, Deshpande MR, Satyanarayana S, et al. Evaluation of TB case finding through systematic contact investigation, Chhattisgarh, India. Tuberculosis Research and Treatment. 2015; 670167. Available at www.hindawi.com/journals/trt/2015/670167/ (accessed on 21 July 2017). [Google Scholar] |
| 10. | Singh J, Sankar MM, Kumar S, Gopinath K, Singh N, Mani K, et al. Incidence and prevalence of tuberculosis among household contacts of pulmonary tuberculosis patients in a peri-urban population of South Delhi, India. PLoS One 2013; 8: e69730. [Google Scholar] |
| 11. | Gupta M, Saibannavar AA, Kumar V. Household symptomatic contact screening of newly diagnosed sputum smears positive tuberculosis patients-An effective case detection tool. Lung India 2016; 33: 159-62. [Google Scholar] |
| 12. | Chatla C, Jaju J, Achanta S, Samyuktha R, Chakramahanti S, Purad C, et al. Active case finding of rifampicin sensitive and resistant TB among household contacts of drug resistant TB patients in Andhra Pradesh and Telangana states of India-A systematic screening intervention. Indian J Tuberc 2018; 65: 218-24. [Google Scholar] |
| 13. | Nair D, Rajshekhar N, Klinton JS, Watson B, Velayutham B, Tripathy JP, et al. Household contact screening and yield of tuberculosis cases-A clinic-based study in Chennai, South India. PLoS One 2016; 11: e0162090. [Google Scholar] |
| 14. | Bendle SS, Goyal RC. Evaluation of household contacts of MDR-TB cases in Wardha district, Maharashtra, India. Int J Curr Res Aca Rev 2015; 3: 26-31. [Google Scholar] |
| 15. | Chinnakali P, Thekkur P, Ramaswamy G, Selvaraj K. Active screening for tuberculosis among slum dwellers in selected urban slums of Puducherry, South India. Ann Trop Med Public Health 2016; 9: 295. [Google Scholar] |
| 16. | Prasad BM, Satyanarayana S, Chadha SS. Lessons learnt from active tuberculosis case finding in an urban slum setting of Agra city, India. Indian J Tubercl 2016; 63: 199-202. [Google Scholar] |
| 17. | Dholakia Y, Mistry N. Active tuberculosis case finding in a migrant slum community, Mumbai, India. Int J Tuberc Lung Dis 2016; 20: 1562. [Google Scholar] |
| 18. | Meundi AD, Meundi MD, Dhabadi BB, Ismail MI, Amruth M, Kulkarni AG. Prevalence of pulmonary tuberculosis among inmates and staff of three Indian prisons. Br J Med Med Res 2016; 11: 1-6. [Google Scholar] |
| 19. | Prasad BM, Thapa B, Chadha SS, Das A, Babu ER, Mohanty S, et al. Status of tuberculosis services in Indian prisons. Int J Infect Dis 2017; 56: 117-21. [Google Scholar] |
| 20. | Mallick G, Shewade HD, Agrawal TK, Kumar AM, Chadha SS. Enhanced tuberculosis case finding through advocacy and sensitisation meetings in prisons of central India. Public Health Action 2017; 7: 67-70. [Google Scholar] |
| 21. | Mave V, Nimkar S, Prasad H, Kadam D, Meshram S, Lokhande R, et al. Tuberculosis screening among persons with diabetes mellitus in Pune, India. BMC Infect Dis 2017; 17: 388. [Google Scholar] |
| 22. | Srinivas Rao MS, Shridhar M, Pavani K, Vinayaraj EV, Manick Dass S. Screening of tuberculosis in diabetic patients at a tertiary care hospital in Hyderabad. Indian J Microbiol Res 2015; 2: 220. [Google Scholar] |
| 23. | India Diabetes Mellitus-Tuberculosis Study Group. Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Health 2013; 18: 646-54. [Google Scholar] |
| 24. | Jali MV, Mahishale VK, Hiremath MB. Bidirectional screening of tuberculosis patients for diabetes mellitus and diabetes patients for tuberculosis. Diabetes Metab J 2013; 37: 291-5. [Google Scholar] |
| 25. | Rao KG, Aggarwal AN, Behera D. Tuberculosis among physicians in training. Int J Tuberc Lung Dis 2004; 8: 1392-4. [Google Scholar] |
| 26. | Dolla C, Padmapriyadarsini C, Pradeep Menon A, Muniyandi M, Adinarayanan S, Sekar G, et al. Tuberculosis among the homeless in Chennai city, South India. Trans R Soc Trop Med Hyg 2017; 111: 479-81. [Google Scholar] |
| 27. | Marimuthu P. Tuberculosis prevalence and socio-economic differentials in the slums of four metropolitan cities of India. Indian J Tuberc 2016; 63: 167-70. [Google Scholar] |
| 28. | Kadri AM, Bhagyalaxmi A, Lala MK, Jivrajini P, Vidhani M, Patel T. An epidemiological study of prevalence of tuberculosis in the urban slum area of Ahmedabad city. Indian J Community Med 2003; 28: 122-4. [Google Scholar] |
| 29. | Chinnakali P, Ramakrishnan J, Vasudevan K, Gurumurthy J, Upadhyay RP, Panigrahi KC. Level of awareness about tuberculosis in urban slums: Implications for advocacy and communication strategy planning in the national program. Lung India 2013; 30: 139-42. [Google Scholar] |
| 30. | Banu S, Rahman MT, Uddin MK, Khatun R, Ahmed T, Rahman, et al. Epidemiology of tuberculosis in an urban slum of Dhaka City, Bangladesh. PloS One 2013; 8: e77721. [Google Scholar] |
| 31. | Sekandi JN, Neuhauser D, Smyth K, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis 2009; 13: 508-13. [Google Scholar] |
| 32. | Pronyk PM, Joshi B, Hargreaves JR, Madonsela T, Collinson MA, Mokoena O, et al. Active case finding: Understanding the burden of tuberculosis in rural South Africa. Int J Tuberc Lung Dis 2001; 5: 611-18. [Google Scholar] |
| 33. | Ababa Demissie M, Zenebere B, Berhane Y, Lindtjorn B. Rapid survey to determine the prevalence of smear-positive tuberculosis in Addis Ababa. Int J Tuberc Lung Dis 2002; 6: 580-4. [Google Scholar] |
| 34. | Chadha VK, Kumar P, Jagannatha PS, Vaidyanathan PS, Unnikrishnan KP. Average annual risk of tuberculous infection in India. Int J Tuberc Lung Dis 2005; 9: 116-18. [Google Scholar] |
| 35. | Gopinath KG, Siddique S, Kirubakaran H, Shanmugam A, Mathai E, Chandy GM. Tuberculosis among healthcare workers in a tertiary-care hospital in South India. J Hosp Infect 2004; 57: 339-42. [Google Scholar] |
| 36. | Aggarwal AN. Tuberculosis transmission at healthcare facilities in India. Lung India 2009; 26: 33-4. [Google Scholar] |
| 37. | Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole- blood interferon gamma assay with tuberculin skin testing. JAMA 2005; 293: 2746-55. [Google Scholar] |
| 38. | Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis 2011; 17: 488-94. [Google Scholar] |
| 39. | Napoli C, Ferretti F, Di Ninno F, Orioli R, Marani A, Sarlo MG, et al. Screening for tuberculosis in health care workers: Experience in an Italian teaching hospital. Biomed Res Int 2017; 2017: 7538037. [Google Scholar] |
| 40. | Gopinath KG, Siddique S, Kirubakaran H, Shanmugam A, Mathai E, Chandy GM, et al. Tuberculosis among healthcare workers in a tertiary-care hospital in South India. J Hosp Infect 2004; 57: 339-42. [Google Scholar] |
| 41. | National Crime Records Bureau. Prison Statistics of India. Available at www.ncrb.gov.in/StatPublications/PSI/Prison2015/Full/PSI-2015-%2018-11- 2016.pdf (accessed on 20 Nov 2017). [Google Scholar] |
| 42. | Ali S, Haileamlak A, Wieser A, Pritsch M, Heinrich N, Loscher T, et al. Prevalence of pulmonary tuberculosis among prison inmates in Ethiopia, a cross-sectional study. PLoS One 2015; 10: e0144040. [Google Scholar] |
| 43. | O'Grady J, Hoelscher M, Atun R, Bates M, Mwaba P, Kapata N, et al. Tuberculosis in prisons in Sub-Saharan Africa-The need health for improved services, surveillance and control. Tuberculosis (Edinb) 2011; 91: 173-8. et al. Tuberculosis in prisons in Sub-Saharan Africa-The need health for improved services, surveillance and control. Tuberculosis (Edinb) 2011; 91: 173-8.'>[Google Scholar] |
| 44. | Vinkeles Melchers NV, van Elsland SL, Lange JM, Borgdorff MW, van den Hombergh J. State of affairs of tuberculosis in prison facilities: A systematic review of screening practices and recommendations for best TB control. PLoS One 2013; 8: e53644. [Google Scholar] |
| 45. | Kong PM, Tapy J, Calixto P, Burman WJ, Reves RR, Yang Z, et al. Skin-test screening and tuberculosis transmission among the homeless. Emerg Infect Dis 2002; 8: 1280-4. [Google Scholar] |
| 46. | Paquette K, Cheng MP, Kadatz MJ, Cook VJ, Chen W, Johnston JC, et al. Chest radiography for active tuberculosis case finding in the homeless: A systematic review and meta-analysis. Int J Tuberc Lung Dis 2014; 18: 1231-6. [Google Scholar] |
| 47. | Chaudhary BL, Band RM, Yadav P, Kumar M, Rani Y. Death due to tuberculosis in homeless unclaimed population in central Delhi-A retrospective study. Indian J Public Health Res Dev 2013; 4: 28-32. [Google Scholar] |
| 48. | Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med 2011; 9: 81 [Google Scholar] |
| 49. | Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 2007; 23: 3-13. [Google Scholar] |
| 50. | Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lönnroth K, et al. Bi-directional screening for tuberculosis and diabetes: A systematic review. Trop Med Int Health 2010; 15: 1300 [Google Scholar] |
| 51. | Nasa JN, Brostrom R, Ram S, Kumar AM, Seremai J, Hauma M, et al. Screening adult tuberculosis patients for diabetes mellitus in Ebeye, Republic of the Marshall Islands. Public Health Action 2014; 4: S50-2. [Google Scholar] |
| 52. | Fox GJ, Dobler CC, Marks GB. Active case finding in contacts of people with tuberculosis. Cochrane Database Syst Rev 2011; (9): CD008477. [Google Scholar] |
| 53. | Shivaramakrishna HR, Frederick A, Shazia A, Murali L, Satyanarayana S, Nair SA, et al. Isoniazid preventive treatment in children in two districts of South India: Does practice follow policy? Int J Tuberc Lung Dis 2014; 18: 919-24. [Google Scholar] |
| 54. | Pothukuchi M, Nagaraja SB, Kelamane S, Satyanarayana S, Shashidhar, Babu S, et al. Tuberculosis contact screening and isoniazid preventive therapy in a South Indian district: Operational issues for programmatic consideration. PLoS One 2011; 6: e22500. [Google Scholar] |
| 55. | Chang KC, Leung CC, Tam CM. Household contact investigation of tuberculosis in low-income and middle-income countries: Public-health impact. Lancet Infect Dis 2009; 9: 3-4 [Google Scholar] |
| 56. | Jia Z, Cheng S, Ma Y, Zhang T, Bai L, Xu W, et al. Tuberculosis burden in China: A high prevalence of pulmonary tuberculosis in household contacts with and without symptoms. BMC Infect Dis 2014; 14: 64. [Google Scholar] |
| 57. | Liu E, Cheng S, Wang X, Hu D, Zhang T, Chu C, et al. A systematic review of the investigation and management of close contacts of tuberculosis in China. J Public Health (Oxf) 2010; 32: 461-6. [Google Scholar] |
| 58. | Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med 2014; 12: 216. [Google Scholar] |
| 59. | Murray CJ, Salomon JA. Expanding the WHO tuberculosis control strategy: rethinking the role of active case-finding. Int J Tuberc Lung Dis 1998; 2 (9 Suppl 1):S9-S15. [Google Scholar] |
Fulltext Views
6,463
PDF downloads
2,487




