Translate this page into:
Anaplastic large cell lymphoma presenting as an endobronchial mass with lung involvement in an adolescent boy
Correspondence to RACHNA SETH
To cite: Moothedath AW, Seth R, Kakkar A, Sharma MC, Sahoo D. Anaplastic large cell lymphoma presenting as an endobronchial mass with lung involvement in an adolescent boy. Natl Med J India 2022;34: 343–6.
Abstract
Primary endobronchial presentation of anaplastic large cell lymphoma is rare in the paediatric age group. We present a 12-year-old boy with breathlessness, fever, cough and weight loss, who was misdiagnosed as a case of tuberculosis and started on antitubercular therapy, which showed no improvement. Chest X-ray showed a completely opacified left hemithorax and chest computed tomography revealed a mass encircling the left main bronchus with collapse– consolidation of the left lung. Fibreoptic bronchoscopy revealed a growth in the left main bronchus. Subsequently, fine-needle aspiration cytology and biopsy from the mass confirmed it to be a malignancy consistent with anaplastic large cell lymphoma. Metastatic work-up revealed no other sites of involvement. Chemotherapy resulted in rapid and complete regression of the tumour. No evidence of local or distant recurrence was reported after 18 months of follow-up. Clinicians and pathologists should be aware of this presentation as prompt diagnosis and treatment can give promising results. This case highlights the importance of timely tissue diagnosis in patients with non-resolving pyrexia and organ lesions on imaging.
INTRODUCTION
Non-Hodgkin lymphoma (NHL) of the lung comprises 3.6% of extranodal lymphomas and around 0.3% of primary lung neoplasms.1 Paediatric NHL most commonly affects the lymph nodes and may be associated with mediastinal involvement and hepatosplenomegaly, with extranodal sites of involvement including the skin, bone, muscle and lung parenchyma.2 Anaplastic large cell lymphoma (ALCL), which represents 15%–20% of paediatric NHLs, occurs most commonly in boys and girls and young adults with a bimodal age distribution.3 When ALCL involves the lung, it is most commonly the result of advanced disseminated disease. It is rare for ALCL to present as an endobronchial disease.
We discuss a 12-year-old boy with primary lung ALCL.
THE CASE
A 12-year-old boy presented to our outpatient department with complaints of exertional breathlessness for 6 months, recurrent episodes of dry cough and intermittent fever for 4 months. There was a history of occasional haemoptysis and expectoration of cast-like material in the sputum. The boy had lost about 20 kg during his illness. There was no history of contact with a patient of tuberculosis. Past and family history was uneventful. A chest X-ray (Fig. 1a) showed a completely opacified left hemithorax with an ipsilateral mediastinal shift. A contrast-enhanced computed tomography (CECT) scan of the thorax showed a mass encircling the left main bronchus with collapse–consolidation of the left lung (Fig. 2a). Subsequently, the boy underwent fibreoptic bronchoscopy, which confirmed a left main bronchial growth appearing as a caseous material adherent to the bronchial wall (Fig. 3). Biopsy of the endobronchial lesion and bronchoalveolar lavage was done. The histopathology showed only necrotic tissue; stain for acid-fast bacilli and the mycobacteria growth indicator tube (MGIT) system was negative. During this time, he received antitubercular therapy for 4 months and dexamethasone for 2 months for suspected endobronchial tuberculosis without clinical improvement. Subsequent to these investigations, he was transferred to our hospital in view of the increasing severity of his symptoms.
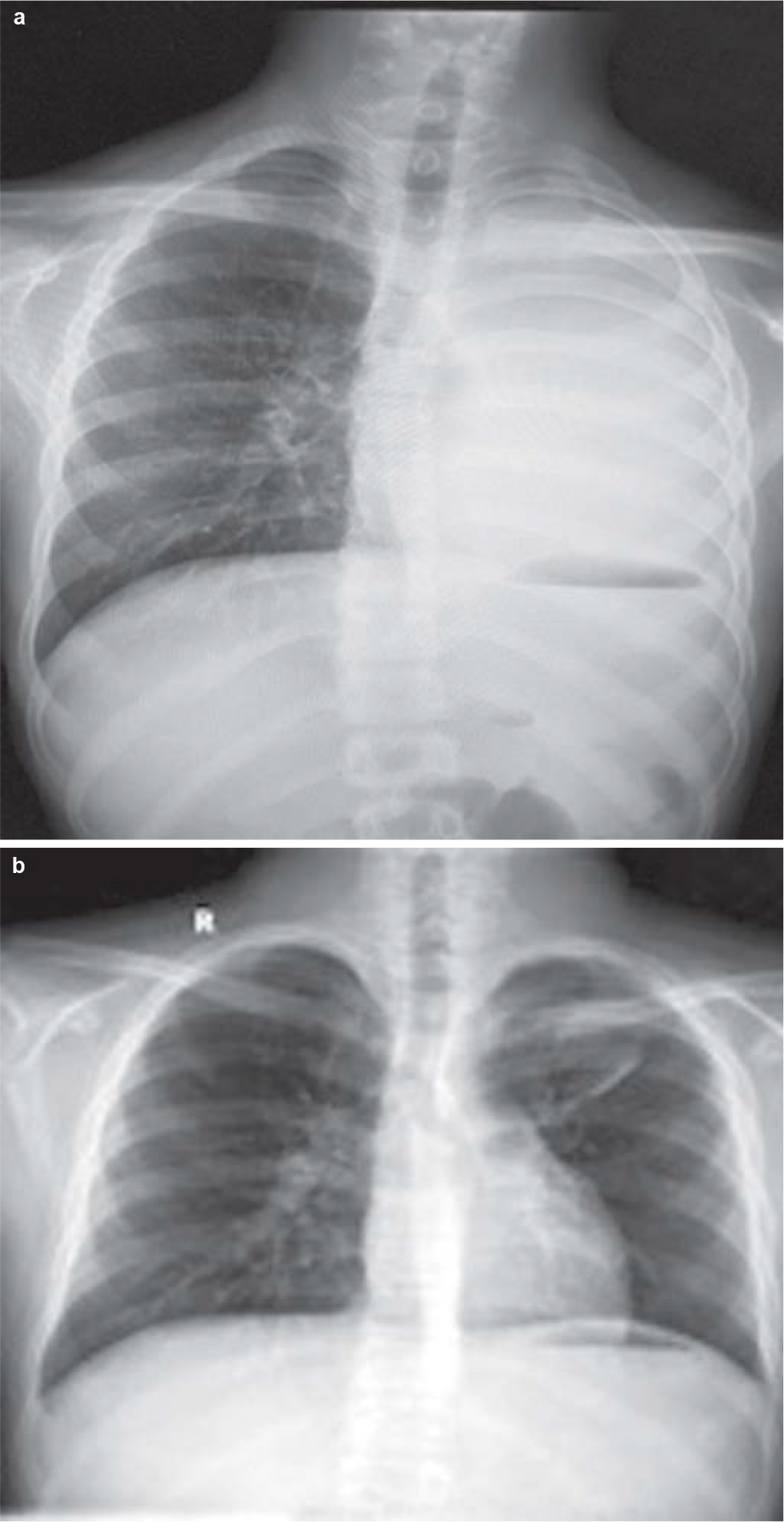
- (a) Chest X-ray showing an opaque appearance of the left lung with ipsilateral mediastinal shift. The left main bronchus is not separately visible; (b) post-treatment chest X-ray showing disappearance of left-sided mass lesion and complete expansion of the left lung
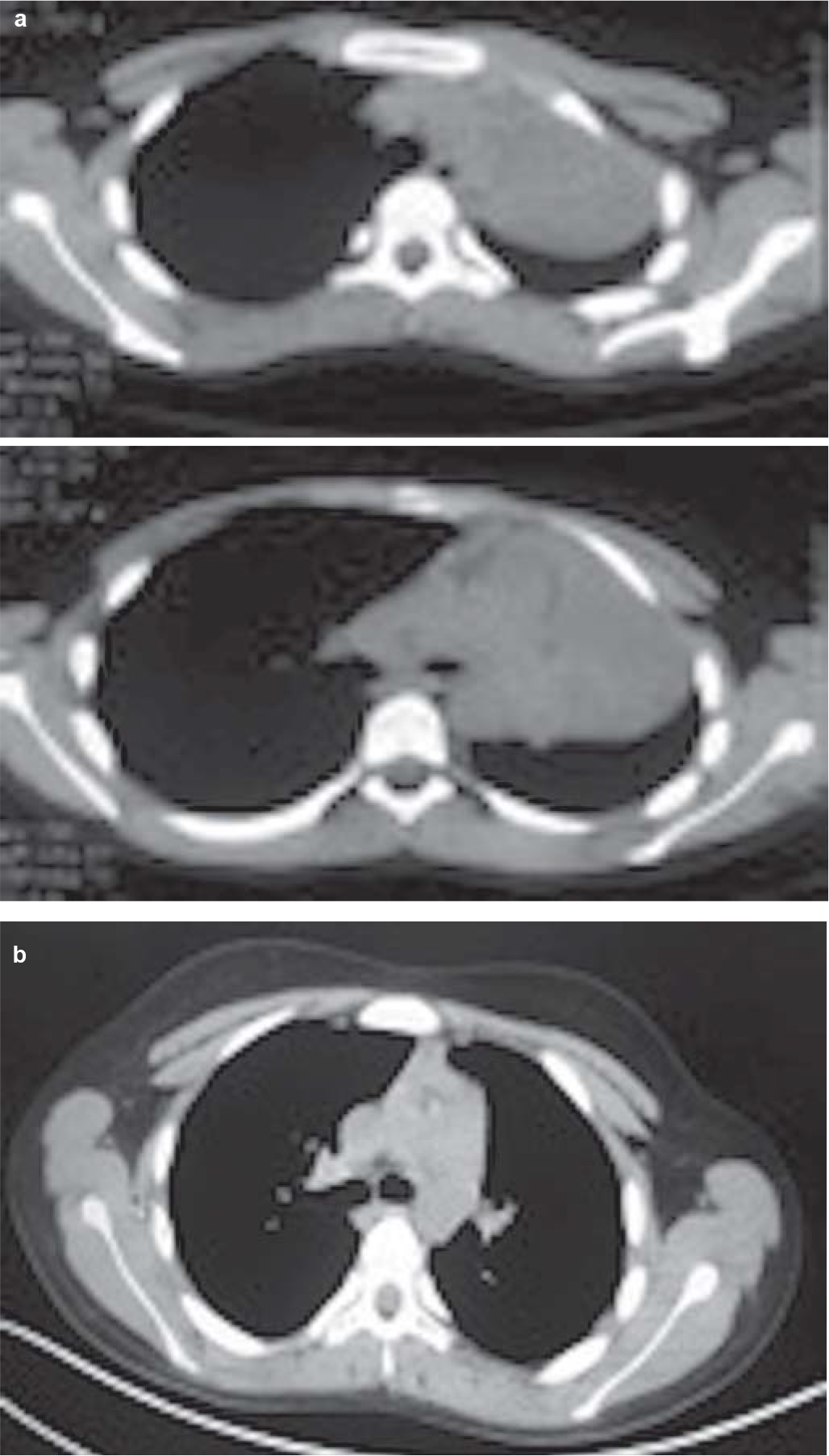
- (a) Computed tomography scan of the chest showing mass encircling left main bronchus with collapse–consolidation of the left lung; (b) following treatment showing disappearance of left-sided mass lesion and complete expansion of the left lung
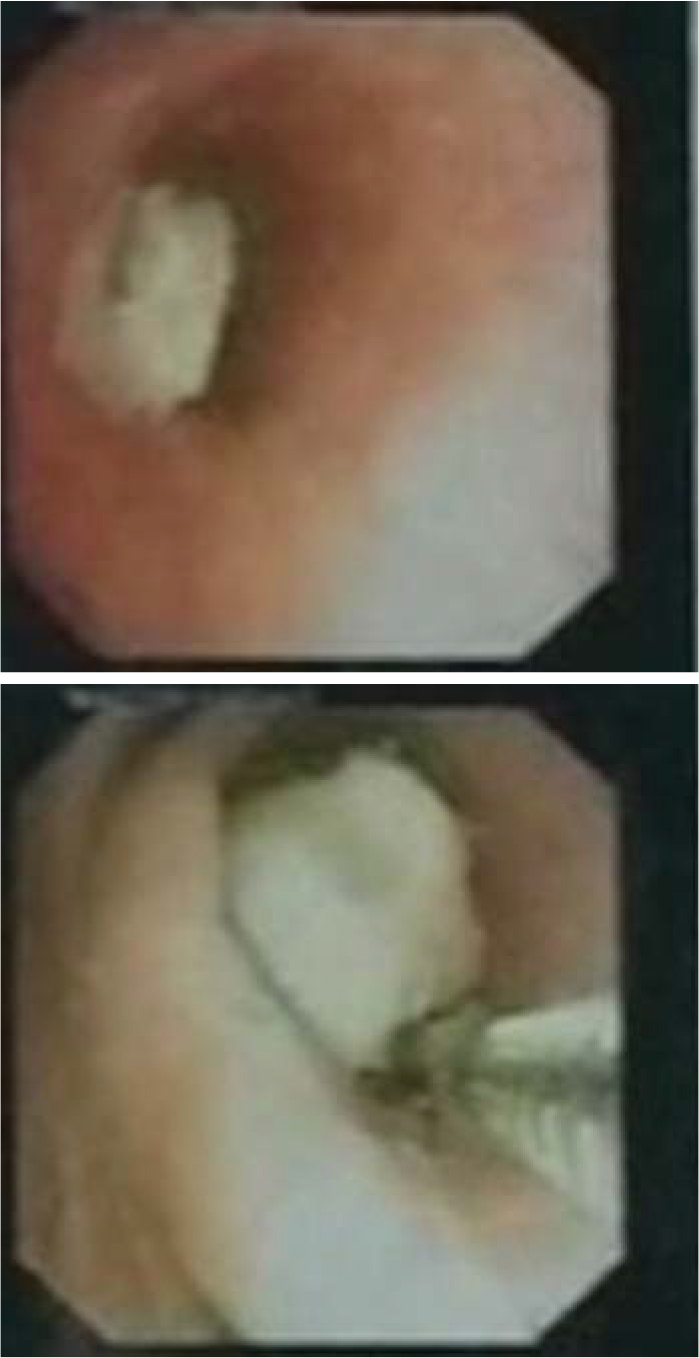
- Bronchoscopic image of the tumour. The left main bronchus is filled with an adherent caseous material (a) obstructing it almost completely (b)
The boy was admitted to our hospital with complaints of fever, tachycardia, tachypnoea, pallor and weight loss. Chest examination showed marked decreased air entry throughout the left lung fields. There was no lymphadenopathy/hepatosplenomegaly. The clinical possibilities were drug-resistant tuberculosis, malignancies (lymphoma and carcinoid) and connective tissue disorders including vasculitis syndromes and sarcoidosis. Fine needle aspiration cytology (FNAC) (Figs 4a–f) from the left hilar mass lesion showed dispersed atypical large lymphoid cells with a moderate amount of cytoplasm and large pleomorphic nuclei. Few hallmark cells with kidney- and doughnut-shaped nuclei were present. Immunocytochemistry performed on de-stained smears for anaplastic lymphoma kinase (ALK) and CD30 showed positivity in some malignant cells, suggesting the diagnosis of ALCL. Following the suspicion of malignancy on FNAC, antitubercular therapy was stopped; steroids were also gradually tapered and discontinued. Ultrasonography-guided biopsy (Figs 5a–f) from the left hilar mass lesion showed similar cells infiltrating fibrocollagenous tissue, which were immunopositive for ALK, CD30, epithelial membrane antigen and CD43. Hence, the diagnosis of primary lung ALK-positive ALCL was confirmed.
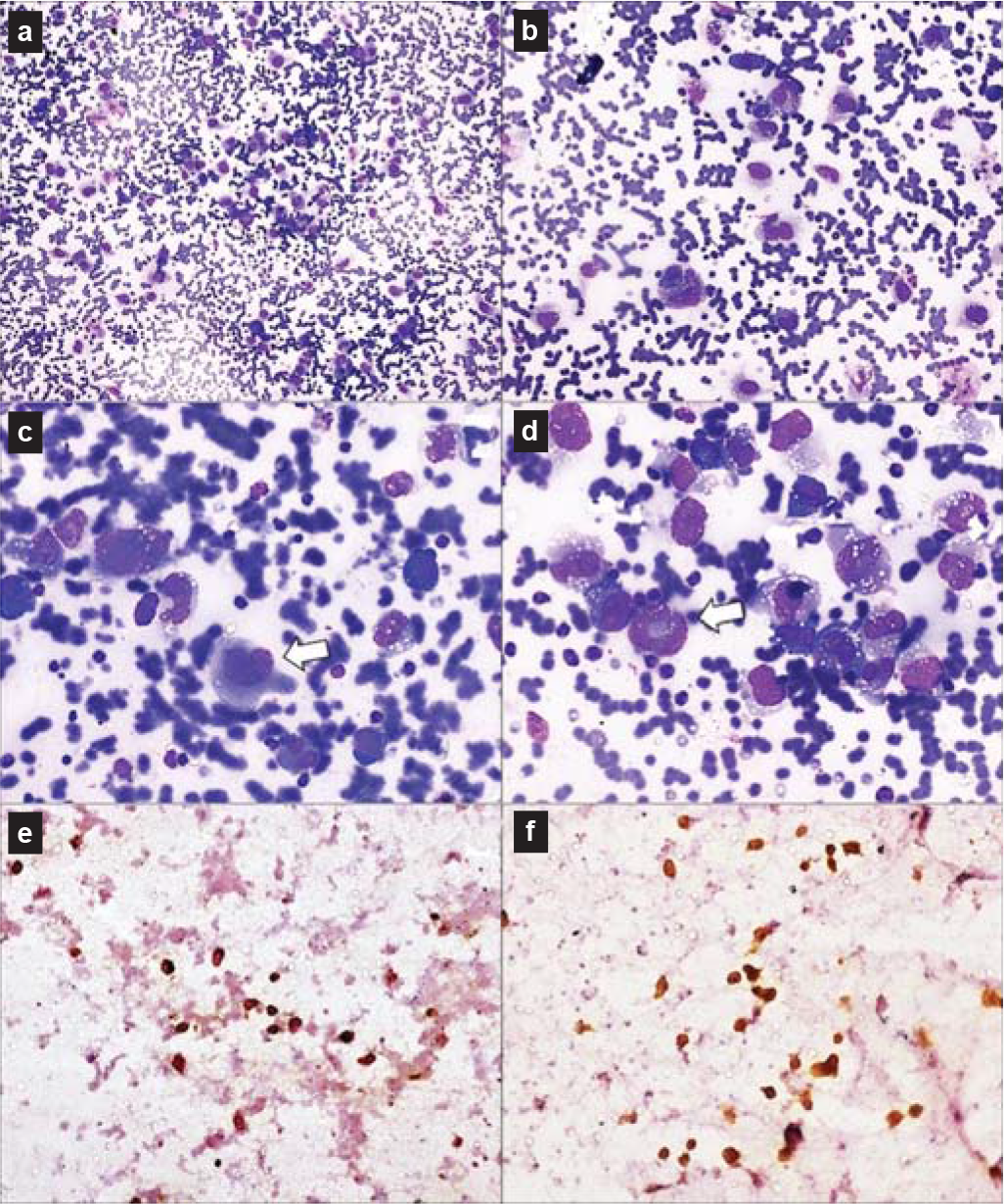
- Fine-needle aspiration cytology from hilar mass showing atypical large lymphoid cells with moderate amount of cytoplasm and large pleomorphic nuclei (a and b). Few hallmark cells (white arrows) with kidney- and doughnut-shaped nuclei were present (c and d). Tumour cells were immunopositive for anaplastic lymphoma kinase (e) and CD30 (f)
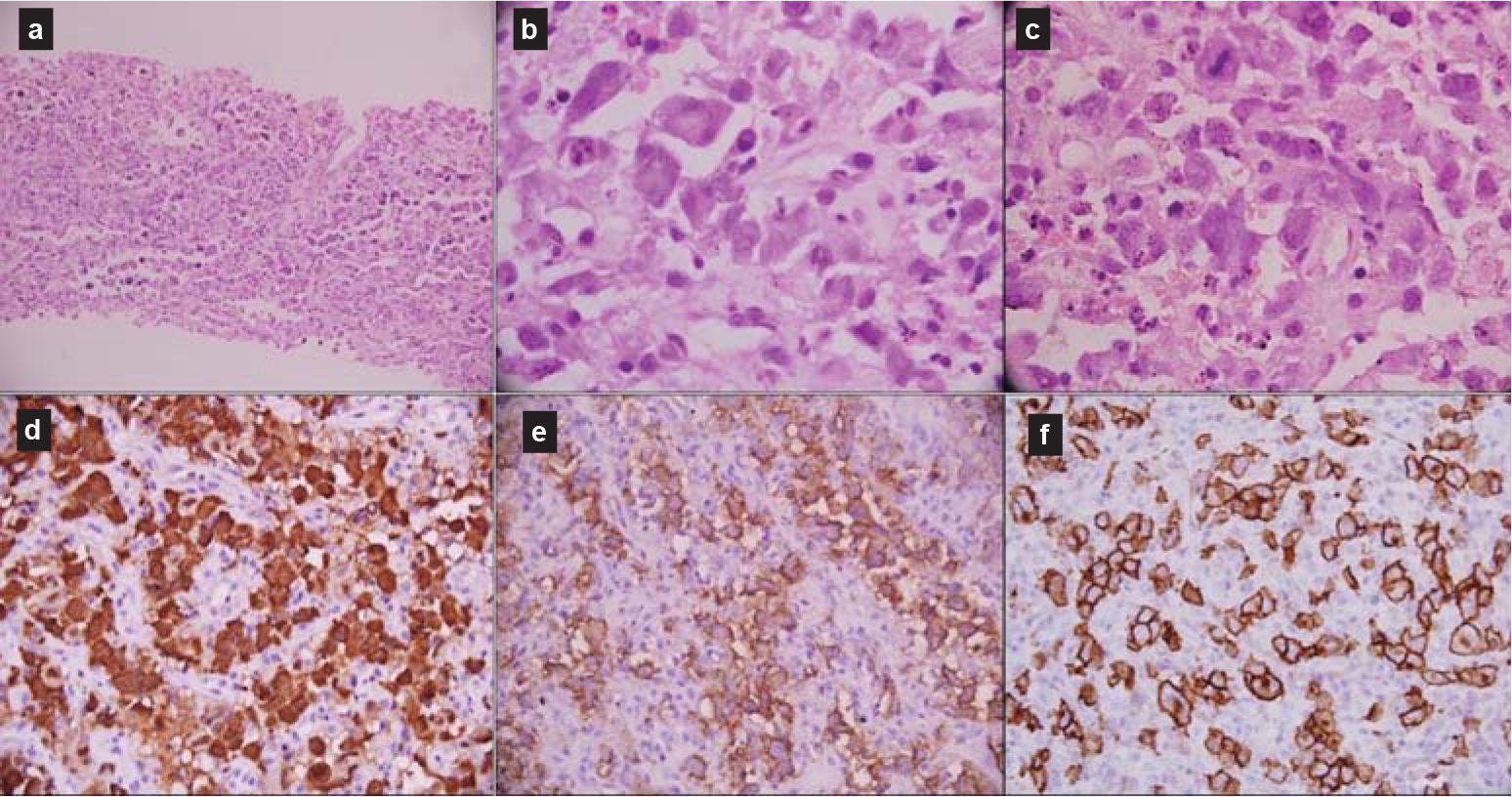
- Core-needle biopsy showed atypical large lymphoid cells (a) with moderate-to-abundant cytoplasm and enlarged vesicular nuclei (b); mitoses were frequent (c). The large cells were immunopositive for anaplastic lymphoma kinase (d), epithelial membrane antigen (e) and CD30 (f)
Staging work-up including cytology of the cerebrospinal fluid and bilateral bone marrow aspiration/biopsy was negative for involvement by ALCL. Fluorodeoxyglucose positron-emission tomography–computed tomography (FDG PET-CT) showed no other sites of involvement (Fig. 6a). The patient was started on BFM-NHL 90 protocol comprising multi-agent intensive chemotherapy including prophylaxis for the central nervous system. He was in complete remission after 6 cycles of chemotherapy. The chest X-ray, CECT chest (Figs 1b and 2b) and FDG PET-CT (Fig. 6b) after treatment showed complete resolution of the mass and disappearance of the metabolic activity. The boy is doing well after more than 18 months of follow-up.
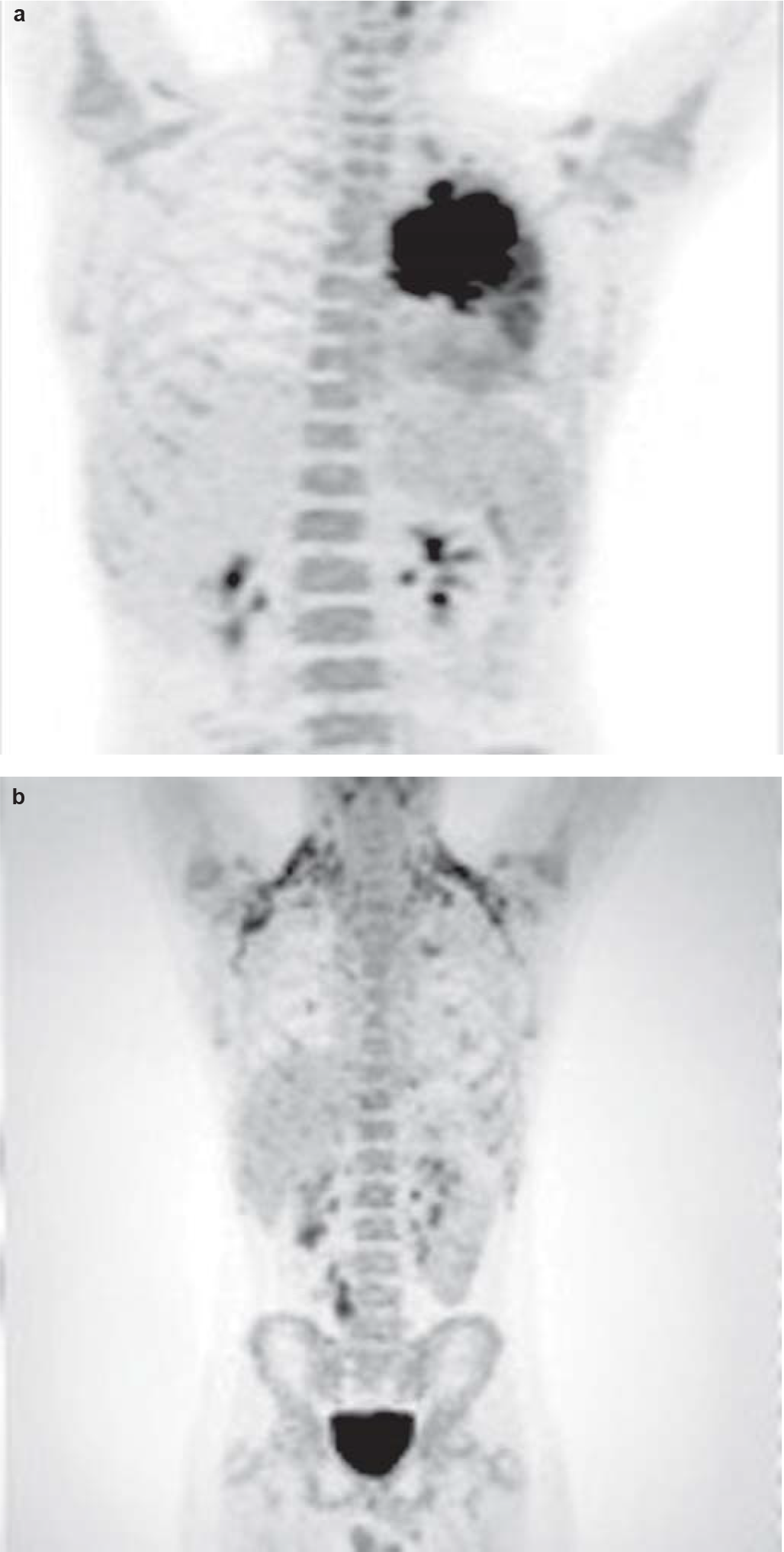
- Maximum intensity projection of fluorodeoxyglucose positron-emission tomography image (a) showing left lung mass. No lesions were found elsewhere; (b) fluorodeoxyglucose positron-emission tomography–computed tomography images after completion of chemotherapy showing resolution of left-sided mass lesion
DISCUSSION
Endobronchial tumours are extremely rare in children/ adolescents constituting only 0.2% of all malignancies. These may be benign such as hamartoma, vascular tumours and papilloma or malignant such as bronchial adenoma, carcinoid, mucoepidermoid carcinoma and adenoid cystic carcinoma.4 Carcinoid is the most common malignant endobronchial tumour,4–6 followed by mucoepidermoid carcinoma. Lymphoma rarely presents as an endobronchial lesion. ALCL is a T cell NHL which includes primary cutaneous and systemic ALCL. Skin is also the most common site of extranodal involvement in ALCL. Hallmarks of ALCL include immunopositivity for ALK protein and ALK gene rearrangements. ALK expression is seen in about 80% of cases of ALCL via chromosomal translocation. Other immunohistochemical (IHC) markers characteristically expressed by ALCL tumour cells are CD30 and epithelial membrane antigen (EMA).
Although lung is one of the sites described in ALCL, endobronchial involvement by ALCL has not been frequently reported. Pulmonary involvement of ALCL occurs as a result of dissemination in 12% of all cases (secondary), and primary endobronchial ALCL is extremely rare.1 The first case of primary isolated endobronchial ALCL in children was described by Guerra et al.2 in 2006. Although NHL presenting as an endobronchial tumour has been reported in the adult population, to the best of our knowledge, only 6 case reports have described primary endobronchial ALCL without evidence of disease elsewhere in children in the English literature2,3,7–10 Three other children with endobronchial ALCL were also described but they had evidence of extrapulmonary disease.11–13 Endobronchial ALCL usually presents clinically with progressive dyspnoea and atelectasis of lung parenchyma, as was seen in our patient. Diagnosing ALCL can be a challenge for both the pathologist and the clinician, but with the current widespread availability of IHC markers and cytogenetic studies, accurate diagnosis can be made in a majority of cases.
The main symptoms of the disease are frequently non-specific, with progressive dyspnoea, fever, cough, weight loss, wheeze and occasionally haemoptysis. It can potentially mimic refractory asthma or tuberculosis in some cases. Hence, paediatricians should suspect bronchial tumour in patients who do not respond to bronchodilator or antitubercular therapy. Chest X-ray shows a lung collapse in nearly 50% of patients with endobronchial involvement and a mass or hilar shadow in 20% of cases.14 Cavitating lung nodules can also be seen as a presenting feature of pulmonary involvement by lymphoma.15
Although a majority of malignant endobronchial tumours require open surgical excision, endoscopic excision may be suitable for selected cases of bronchial carcinoid and laser therapy using rigid bronchoscope for benign conditions such as tracheobronchial granulation tissue and granular cell tumour. Lung ALCL in boys and girls responds well to chemotherapy, akin to ALCL in other regions with the majority achieving complete remission. However, recurrence rates may be as high as 40% and predominantly occur within months of completion of treatment.2 Our patient showed an excellent response to intensive multi-agent chemotherapy and attained clinical and radiological complete remission within 6 months. He is on regular follow-up in our cancer survivor clinic and is doing well.
Conclusion
Bronchial ALCL must be considered in the differential diagnosis in case of airway obstruction/mass lesion/collapse– consolidation, especially in young patients. Due to their rare occurrence, such lesions remain a diagnostic challenge and require a high degree of clinical suspicion for accurate diagnosis and to initiate early appropriate treatment. On clinical grounds, ALCL is a relatively infrequent tumour. Endobronchial lesions in NHL are the rarest intrathoracic manifestation of ALCL even in advanced widely disseminated disease.
Conflicts of interest
None declared.
References
- Primary anaplastic large cell lymphoma of the lung: A clinicopathologic study of five patients. Mod Pathol. 2000;13:1285-92.
- [CrossRef] [PubMed] [Google Scholar]
- Primary endobronchial anaplastic large cell lymphoma in a pediatric patient. P R Health Sci J. 2006;25:159-61.
- [Google Scholar]
- Endobronchial ALK positive anaplastic large-cell lymphoma resembling asthma in a 13-year-old girl. J Pediatr Hematol Oncol. 2013;35:e4-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and outcomes of malignant pediatric lung neoplasms. J Surg Res. 2009;156:224-30.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant endobronchial lesions of adolescence. Pediatr Radiol. 1992;22:563-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pathologic quiz case: A 17-year-old adolescent girl with a short history of dyspnea. Endobronchial, anaplastic large cell lymphoma, T-cell phenotype. Arch Pathol Lab Med. 2003;127:e430-1.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated pediatric endobronchial primary anaplastic large cell lymphoma. J Pediatr Surg Case Rep. 2014;2:66-9.
- [CrossRef] [Google Scholar]
- Anaplastic large cell lymphoma presenting as bilateral endobronchial tumor in a young boy. Lung India. 2018;35:66-9.
- [CrossRef] [PubMed] [Google Scholar]
- Solitary endobronchial anaplastic large cell lymphoma revealed by atelectasis: About a pediatric observation. Int J Cancer Oncol. 2018;5:13-15.
- [CrossRef] [Google Scholar]
- Anaplastic large cell lymphoma presenting as an endobronchial polypoid mass. Leuk Lymphoma. 2012;53:2078.e9.
- [CrossRef] [PubMed] [Google Scholar]
- Anaplastic large-cell lymphoma presenting as an endobronchial polypoid tumor. J Clin Oncol. 2008;26:4845-7.
- [CrossRef] [PubMed] [Google Scholar]
- Combined chemotherapy and tracheobronchial stenting for life-threatening airway obstruction in a child with endobronchial non-Hodgkin lymphoma. Pediatr Hematol Oncol. 2004;21:725-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary involvement in pediatric lymphoma. Pediatr Radiol. 2004;34:120-4.
- [CrossRef] [PubMed] [Google Scholar]




