Translate this page into:
Burden of malaria during pregnancy in perennial transmission settings of two densely forested and remote blocks (Baihar and Birsa) of district Balaghat, Madhya Pradesh, central India
Correspondence to RAJASUBRAMANIAM SHANMUGAM; shanmugamsweet@gmail.com
[To cite: Jain V, Sharma RK, Shukla MM, Khosla KK, Singh N, Shanmugam R. Burden of malaria during pregnancy in perennial transmission settings of two densely forested and remote blocks (Baihar and Birsa) of district Balaghat, Madhya Pradesh, central India. Natl Med J India 2023;36:351–7. DOI: 10.25259/NMJI_535_21]
Abstract
Background
Malaria in pregnancy (MIP) is a major public health problem due to the vulnerability of pregnant women to infections, resulting in adverse maternal/foetal outcomes in endemic areas.
Methods
We did a field-based study to assess the burden of MIP (prevalence at the time of enrolment and follow-up) and to identify risk factors for MIP in the Birsa and Baihar blocks of district Balaghat in Madhya Pradesh, which have perennial malaria transmission. Malaria screening (during 2015–2017) was done by microscopy and bivalent rapid diagnostic test (SD Bioline RDT, malaria antigen Plasmodium falciparum/Plasmodium vivax Pf/Pv). Dried blood spots were used for haemoglobin estimation. Sociodemographic details with past and present pregnancy status were obtained. A subset of pregnant women were followed up for malaria during pregnancy. Women were also screened for malaria post delivery. Malaria treatment was given as per the National Guidelines of 2013. Multivariate analysis was done to assess independent risk factors for malaria.
Results
A total of 1728 pregnant women were screened, of which 1651 were included in the final analysis. Malaria prevalence at first screening was 23.4% (Pf 88%). Prevalence and Pf parasitaemia both were significantly higher among primigravid (G1) compared to multigravid (G>2; p value 0.012 and 0.019, respectively). Pregnant women of the Baiga ethnic group were more likely to have malaria compared to those belonging to the Gond group (OR [95% CI]; 2.4 [1.7–3.4]; p<0.00001) and non-indigenous group (OR [95% CI]; 8.3 [3.9–19.7]; p<0.00001). Primigravid status of women, first and second trimester of pregnancy, women belonging to indigenous ethnic tribal group and cash crop insufficiency for whole year (a socioeconomic indicator) in the family were the independent risk factors for malaria.
Conclusion
MIP is a major public health problem in forested tribal settlements of Birsa and Baihar blocks of Balaghat district in Madhya Pradesh and requires immediate intervention.
INTRODUCTION
Women during their first and second pregnancy (especially up to second trimester) are more susceptible to malaria infection compared to non-pregnant women of the same reproductive age group.1 The main attributing factor for the burden of malaria in pregnancy (MIP) is poor/declining immunity due to changes in hormonal balance in women, resulting in susceptibility to various complications in both mother and the foetus.2,3 Worldwide, 125 million women are at risk of acquiring malaria infection during pregnancy.4
Plasmodium falciparum (Pf) is a major cause of morbidity and mortality among pregnant women and infants in subSaharan Africa, whereas in Southeast Asia and South America, P. vivax (Pv) also contributes in a large manner to this burden.1,5,6 Diagnosis and treatment of malaria in pregnancy (mainly Pf), especially placental malaria is challenging due to parasite sequestration, excess white blood cells and thick viscous placental blood in a smear.7,8
During pregnancy, adverse outcomes due to malaria exist for both the foetus and newborn (abortion, intrauterine foetal death, preterm deliveries, low birth weight, congenital malaria and neonatal death).5,9 Often, mothers experience severe outcomes (severe anaemia, severe malaria involving vital organs such as brain and lungs, and maternal death).1,9–12 About 40% of low birth weight newborns (LBW) in malaria-endemic areas may be attributable to malaria and are responsible for 62 000 to 363 000 newborn deaths each year.13,14
In 2015, India was the third highest contributor of malaria globally after Nigeria and Democratic Republic of Congo. However, a 24% decline in malaria cases was noted in 2017 coinciding with a decline in malaria in Southeast Asia.15,16 In the past two-and-a-half decades, species shift has been observed in many parts of India with Pf overlapping with Pv.17 Previous hospital and field-based studies from India have shown wide variations in malaria prevalence ranging from 1.8% to 58% among pregnant women in different regions with high risk for adverse outcomes.18–23 However, field-based burden estimation of MIP specifically from perennial malaria transmission settings in India is lacking. This will enable planning for better combat strategies against malaria among pregnant women in similar settings.
Previously, very high malaria prevalence (mainly asymptomatic) among different age groups with increasing trends of malaria (>30% infant positivity rate) in the same study area (district Balaghat) has been reported.24,25 However, no information about MIP is available for this region. We did this field-based investigation on MIP in two blocks of district Balaghat, Madhya Pradesh, central India to characterize the epidemiology and burden of MIP (in terms of infection prevalence with gravidity status during the antenatal and puerperium period, and identification of risk factors for malaria) to tailor specific policies for strengthening malaria control in densely forested and stable malaria transmission areas of India.
METHODS
Site description
The study was carried out in Baihar and Birsa blocks of district Balaghat, Madhya Pradesh, central India. More than 70% of the population belongs to ethnic communities, mainly Gonds and Baigas. Dwellings of these communities are made up of mud and the roof is mud-tiled on a wooden structural base. Roof of the Baigas houses are thatched with straw. This remote area is hilly and covered with thick, dense forest (longitude 80°15' E and 21°84' N). Access to healthcare facilities is poor.
Ethical approval
Our study is encompassed in an institutional review approved project entitled ‘State intervention model for malaria control in a highly malarious district Balaghat, MP by intensified blood surveys and drug administration’.
Definitions
Asymptomatic malaria was defined as absence of fever or history of fever in the presence of the malaria parasite in peripheral blood smear. Anaemia and severe anaemia were defined as previously.23
Screening of pregnant women
Women aged >17 years (in any trimester) were screened during 2015–2017 in the village-based Anganwadis (Integrated Child Development Service [ICDS] centres). Pregnancy confirmation and gestational age calculation were usually done by auxiliary nurse midwives (ANMs) from state departments during the vaccination schedule, using pregnancy test kits. Demographic details including age, current pregnancy record and past reproductive history, fever, etc. were noted. Women were encouraged to take iron and folic acid supplements regularly. Additional clinical measurement of gestational age (fundal height at the time of screening) was not possible. Pregnant women were encouraged to have an institutional delivery.
Peripheral blood investigations
On the spot malaria diagnosis (1304 [79%] pregnant women) was done using bivalent rapid diagnostic tests (RDTs; SD Bioline malaria Ag Pf/Pv). Results of RDTs were available within 20 minutes. In cases where RDT test was not administered due to non-availability, the diagnosis was done using blood smear microscopy (347 [21%] pregnant women). Results of microscopy were available within 48 hours. Blood smears were fixed with methanol and stained using JSB staining protocol.26 Parasitaemia (asexual) counting was done against 200 white blood cells (WBCs) in thick smear. Filter paper blood spots (20 microlitre) were also obtained for haemoglobin estimation by the indirect cyan-methaemoglobin method.
Treatment
If any pregnant women presenting in the second and third trimester were found positive for Pf malaria, they were given artemisinin-based combination therapy (ACT).27 In women who were in the first trimester, quinine sulphate tablets (7 days × 3 tablets) were given and/or were advised to attend the nearest government healthcare centre. If positive for Pv, women were given chloroquine.27 P. malariae (Pm) and other unknown infections were treated by ACT. It took 1–7 days to reach each positive pregnant woman again for treatment if the RDT test could not be administered on the spot. Pregnant women with severe anaemia were brought to the knowledge of Anganwadi workers for necessary treatment.
Follow-up
A subset of pregnant women could be followed up (n=350) for malaria prior to delivery (Fig. 1). Women (n=808) and neonates/infants (n=361) were also screened for malaria after delivery. Only 331 women could be screened for malaria in the puerperium period.
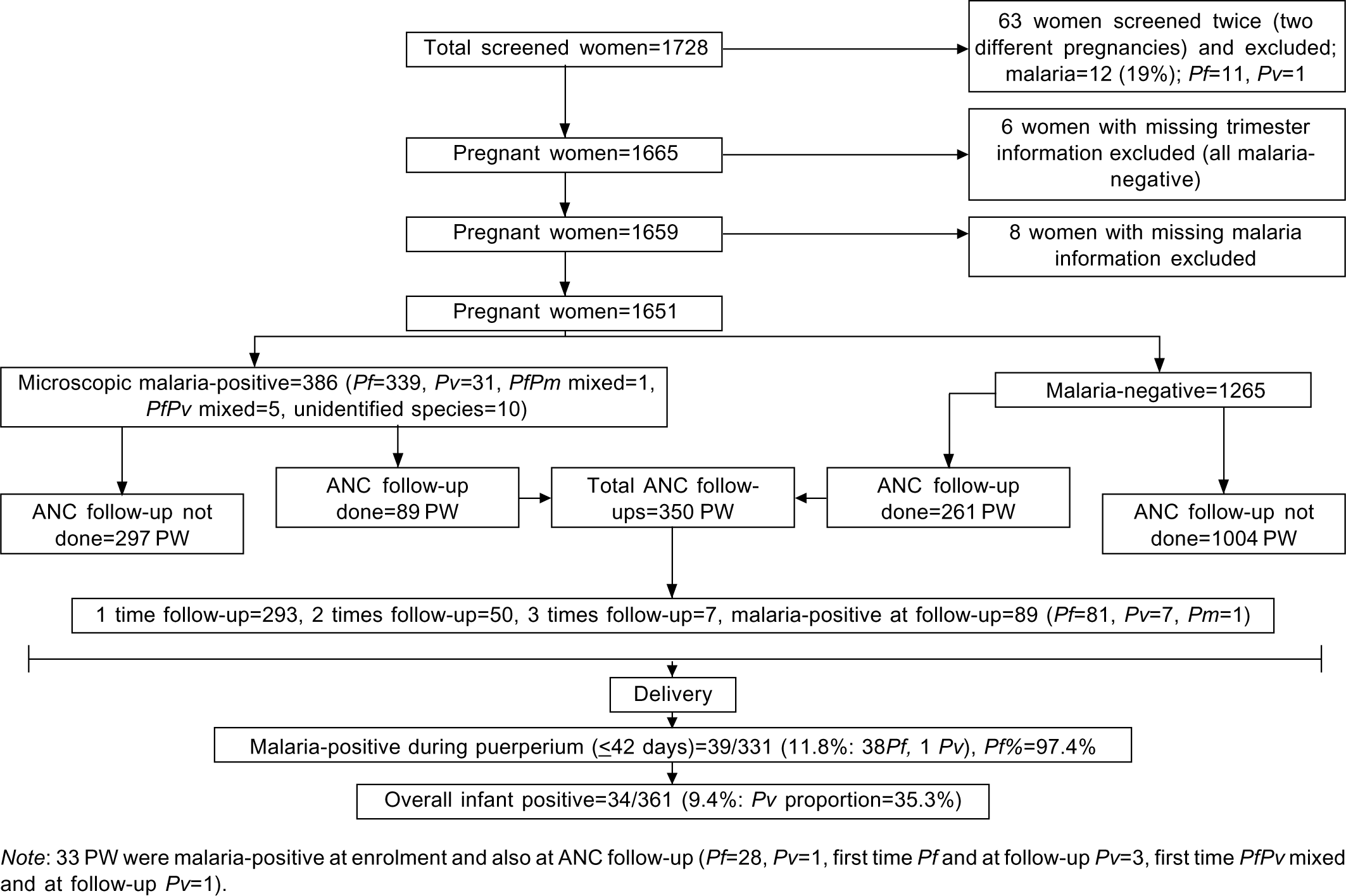
- Flowchart showing malaria screening (2015–2017) of pregnant women (PW)
Data analysis
Pregnant women were identified using a unique number. STATA 12.1 (Texas, USA) software was used for analysis. Categorical variables are presented as numerator/denominator (%) whereas numerical variables (continuous) are presented as median (IQR), number of observations. Data analysis was done using chi-square test for categorical variables and non-parametric Wilcoxon rank-sum (Mann–Whitney) statistical test for numeric variables (for data with non-normal distribution). Univariate and multivariate analyses were done to identify the odds of risk (95% confidence interval) for different factors and their independent association with malaria outcome after binary recoding of various risk factors as 0 (no risk) and 1 (risk).
RESULTS
Baseline characteristics
A total of 1728 pregnant women were screened but due to incomplete records and multiple screening (Fig. 1), 1651 (558 G1 or primigravid and 1093 G≥2 or multigravid) were included in the final analysis. Age (median [IQR]) of pregnant women at the time of first screening was 23 (21–25) years. Thirteen per cent women were screened in their first trimester, 46% in the second trimester and 40% in the third trimester. Overall, the prevalence of anaemia and severe anaemia were 79% and 17%, respectively. Twenty-six (1.6%) women reported to have had two or more previous foetal losses.
Socioeconomic data were collected in a subset of pregnant woman, of the 963 women with a known background of ethnicity, 88.6% belonged to indigenous tribal groups (74.4% were Gonds and 25.6% were Baigas) and the rest belonged to either scheduled caste or general category. Educational information (n=838) revealed that 60.3% women had obtained only primary education or had no schooling, 21.7% studied up to 8th grade and 18% women had education up to high school or above (10th grade or above).
Gravidity and trimester-wise malaria analysis (baseline screening)
Ten per cent (10.3%) of pregnant women reported fever. The overall prevalence of malaria was 23.4% and only 19.5% infections were febrile and/or presented with a history of fever (Table I). Most of the malaria infections were due to Pf (87.8%). The proportion of malaria in the febrile group was significantly higher compared to the afebrile group (42.7% v. 20.2%; OR 95% CI 2.9 [2.1–4.2]; p<0.00001). The prevalence of malaria was significantly higher among G1 compared to G>2 pregnant women (27.1% v. 21.5%; p=0.012; Table I). Women who presented in the first and second trimester had a comparatively higher prevalence of malaria than those who presented in the third trimester (p=0.006 and p<0.0001, respectively).
| Characteristic | Primigravida | Multigravida | Total | p value |
|---|---|---|---|---|
| Number enrolled | 558 | 1093 | 1651 | |
| Median (IQR) age (years) | 20 (20–22) | 25 (23–27) | 23 (21–25) | <0.00001 |
| Median (IQR) Hb (g/dl), n | 9.5 (7.7–10.8), 439 | 9.1 (7.5–10.7), 818 | 9.3 (7.6–10.7), 1257 | 0.025 |
| Anaemia (Hb <11 g/dl) (%) | 344/439 (78.4) | 651/818 (79.6) | 995/1257 (79.2) | 0.61 |
| Severe anaemia (Hb <7 g/dl) (%) | 66/439 (15.0) | 152/818 (18.6) | 218/1257 (17.3) | 0.113 |
| Malaria prevalence (%) | 151/558 (27.1) | 235/1093 (21.5) | 386/1651 (23.4) | 0.012 |
| Fever/fever history during malaria infection (%) | 30/145 (20.7) | 40/214 (18.7) | 70/359 (19.5) | 0.639 |
| Pf prevalence (%) | 141/558 (25.3) | 198/1093 (18.1) | 339/1651 (20.5) | 0.001 |
| Pv prevalence (%) | 7/558 (1.3) | 24/1093 (2.2) | 31/1651 (2.0) | 0.123 |
| Median Pf parasite density (per μl), (95% CI), n | 338.6 (80–960), 101 | 200 (40–480), 125 | 247.3 (78.1–680), 226 | 0.019 |
Pf P. falciparum Pv P. vivax. There were five infections due to mixed PfPv, one infection due to mixed Pf and Pm (P. malariae) and 10 due to unidentified malaria species.
Species-wise analysis revealed that prevalence of Pf malaria was significantly higher among G1 women compared to G>2 women (p=0.001). Prevalence of Pf among G1 women was higher in all the trimesters than G>2 but was statistically significant only in the second trimester (p=0.005). Haemoglobin (Hb) levels (mg/dl) were significantly higher among G1 than G>2 women (p=0.025). However, no significant difference was noted in anaemia/severe anaemia proportions between the two gravida groups (p=0.61 and 0.11, respectively; Table I). Haemoglobin levels were significantly lower among women who were positive for malaria compared to those who were negative (Hb 9.03 [7.4– 10.45]; n=273 v. 9.35 [7.62–10.82]; n=984; p=0.028). Among women with Pf and mixed infections (Pf+Pv/Pm), the prevalence of anaemia and severe anaemia were 85.8% (199/232) and 19.8% (46/232), respectively, whereas prevalence of anaemia among those with Pv infections was 75% (21/28) and severe anaemia was 7.1% (2/28).
The median (IQR) Pf parasite density was significantly higher among G1 women compared to G>2 (p=0.019). Further, trimester-wise analysis of Pf parasite density revealed that during the second trimester, parasitaemia among G1 women was significantly higher compared to G>2 women (p=0.009). The median (IQR) Pf parasite density was higher among those who reported fever at the time of screening compared to those without fever (303.6 [80–783.5] v. 240.0 [74.1–560.8]; p=0.378).
Univariate and multivariate analysis
Univariate and multivariate analyses were done to identify independent risk factors for malaria prevalence in pregnant women. Univariate analysis revealed that primigravid status, presenting trimester (first/second), anaemia, ethnicity (tribe background), education below high school, no bed net availability and insufficiency of cash crop (an economic indicator) for the whole year were significant risk factors for higher malaria prevalence (Table II). On the other hand, on multivariate analysis, factors such as primigravid status of women, first and second trimester, women belonging to indigenous tribal group (ethnicity) and cash crop insufficiency (whole year) were the independent risk factors.
| Variable | Malaria prevalence at enrolment (%) | Odds ratio (95% CI), p value | Adjusted odds ratio, 95% CI, p value |
|---|---|---|---|
| Gravidity (n=1651) | |||
| Multigravida (ref) | 235/1093 (21.5) | 1.4 (1.1–1.7), 0.012 | 1.6 (1.0–2.5), 0.041 |
| Primigravida | 151/558 (27.1) | ||
| Trimester (n=1651) | |||
| Third (ref) | 121/666 (18.2) | 1.7 (1.3–2.1), <0.0001 | 2.0 (1.2–3.3), 0.006 |
| First and second | 265/985 (26.9) | ||
| Anaemia (n=1257) | |||
| No (ref) | 41/262 (15.7) | 1.6 (1.1–2.4), 0.008 | 1.7 (0.9–3.4), 0.101 |
| Yes | 232/995 (23.3) | ||
| Severe anaemia (n=1257) | |||
| No (ref) | 223/1039 (21.5) | 1.1 (0.8–1.5), 0.632 | 0.9 (0.5–1.5), 0.672 |
| Yes | 50/218 (22.9) | ||
| Indigenous tribal group (n=963) | |||
| Non-tribal (ref) | 9/110 (8.2) | 4.4 (2.2–8.9), <0.0001 | 4.0 (1.4–11.8), 0.011 |
| Tribal | 242/853 (28.4) | ||
| Educational background (n=838) | |||
| High school and above (ref) | 26/151 (17.2) | 1.8 (1.1–2.9), 0.010 | 1.7 (0.9–3.2), 0.125 |
| Middle school and below | 188/687 (27.4) | ||
| Bed net ownership (n=836) | |||
| Yes (ref) | 39/209 (18.7) | 1.6 (1.1–2.3), 0.026 | 1.2 (0.7–2.0), 0.542 |
| No | 165/627 (26.3) | ||
| Cash crop sufficiency for whole year (n=871) | |||
| Yes (ref) | 65/339 (19.2) | 1.6 (1.2–2.3), 0.004 | 1.6 (1.0–2.6), 0.039 |
| No | 148/532 (27.8) | ||
| Season (n=1651) | |||
| Winter and Summer (ref) | 229/1108 (20.7) | 1.6 (1.2–2.0), <0.0001 | 1.0 (0.6–1.7), 0.859 |
| Rainy | 157/543 (28.9) | ||
Association of malaria with indigenous tribal group
Comparison of tribal versus non-tribal population of pregnant women for malaria prevalence revealed that the tribes carried significantly higher malaria infections than non-tribals (28.4% v. 8.2%; OR [95% CI] 4.4 [2.2–8.9]; p <0.0001; Table II). Malaria prevalence was higher among pregnant women of the Baiga tribe (93/218; 42.7%) than Gonds (149/635, 23.5%; OR [95% CI] 2.4 [1.7–3.4]; p<0.00001) and non-indigenous women (OR [95% CI] 8.3 [3.9–19.7]; p<0.00001).
Malaria prevalence at follow-up (FU)
Among 350 pregnant women, a total of 414 ANC follow-up visits (140 in G1 and 274 in G>2) were made (Fig. 1). Further, 331 PNC follow-up visits (111 in G1 and 220 in G>2 women: within puerperium period) were also done (Table III). At ANC FU, prevalence of anaemia was 89.9% (median [IQR] Hb 8.6 [7.1– 10.0]; n=268). During PNC follow-up (within puerperium period) anaemia and severe anaemia prevalence were 77.5% and 9.9%, respectively (median [IQR] Hb=9.2 [7.9–10.9]; n=262).
| Characteristic | Primigravida | Multigravida | Total | p value |
|---|---|---|---|---|
| Antenatal | ||||
| Malaria prevalence at follow-up (%) | 41/140 (29.3) | 48/274 (17.5) | 89/414 (21.5) | 0.006 |
| Median (IQR) Pf parasitaemia (per μl), n | 150.5 (40–897.6), 26 | 100 (40–534.5), 36 | 120 (40–676.6), 62 | 0.38 |
| Postnatal (6 weeks) | ||||
| Malaria prevalence, (%) | 13/111 (11.7) | 26/220 (11.8) | 39/331 (11.8) | 0.765 |
| Median (IQR) Pf parasitaemia (per μl), n | 118.8 (40–267.9), 9 | 80 (40–360), 23 | 99.4 (40–271.9), 32 | 0.846 |
Pf P. falciparum. Of 89 malaria infections during antenatal follow-up, malaria proportion due to Pf 91%, Pv 7.9%, Pm (P. malariae) 1.1%. Of 39 malaria infections during postnatal follow-up, malaria proportion due to Pf 97.4%, Pv 2.6%.
During ANC and PNC follow-up >90% infections were due to Pf. Overall malaria prevalence at ANC follow-up was 21.5% (higher in G1 women than G>2 women, p=0.006; Table III). Of 350 pregnant women followed up during ANC, 28 (8%) women again reported Pf malaria (of these 25 women tested for anaemia, 32% showed Hb <7 g/dl) and 5 had Pv infection at follow-up.
During PNC follow-up within the puerperium period (<42 days of delivery) prevalence of malaria was 11.7% among G1 and 11.8% in G>2 women (Table III). Compared to the first time point ANC screening, Pf parasitaemia declined at ANC follow-up (p =0.288) and PNC follow-up within puerperium period (p =0.029). At the time of PNC follow-up 361 infants were also tested for malaria, which revealed a malaria prevalence of 9.4% (Fig. 1).
Additionally, 477 women were followed-up post-puerperium period (>42 days after delivery). Of these, 10.9% (52/477) had Pf infection, 1.7% (8/477) had Pv infection and 0.41% (2/477) had mixed infection (Pf+Pv). From the first ANC screening (386 pregnant women had malaria) to ANC follow-ups, malaria episodes were recorded in a total of 438 women and further including puerperium follow-ups, malaria episodes were registered in 469 pregnant/delivered women in the study area. In this study 56.9% deliveries were conducted at home.
DISCUSSION
Previous studies on the burden of MIP from India were mainly hospital-based and only a few described community-based disease burden. Further, most of the community-based reports were related to outbreak/epidemic investigations. Our study attempted to identify the key gaps in the existing knowledge based on a larger sample size, particularly from remote and densely forested perennial transmission areas, with no or minimal health access. Malaria transmission in such areas is determined by the ecosystem, predominant vector prevalence, insecticide resistance and the presence of drug-resistant parasites.28 In our study 1651 pregnant women were screened for malaria (symptomatic and asymptomatic) and the risk factors for malaria were recorded.
The first recorded evidence of malaria in pregnancy in India was about a century ago, wherein 300 premature births, and 1100 stillbirths, miscarriages and abortions occurred.29 In the two epidemic-based field studies in central India of 274 and 456 pregnant women, respectively, revealed 21%–55% prevalence of malaria (Pf 64%–88%), being especially high in the second trimester (80% pregnant women were also anaemic). Abortion, stillbirths and neonatal deaths were higher in primigravidas being 2.6%–3%, 4%–11% and 2%, respectively among women.21,30 A community-based study of 209 pregnant women from Southeast India showed seasonal parasite-index of 10.8% to 26% with significantly higher infection among primigravida.20 Thus, acute malarial infections are associated with high risk for an adverse pregnancy outcome and therefore it is important to determine and document community-based burden of MIP in areas of different malaria endemicity.
We found malaria prevalence and Pf parasitaemia were significantly higher among primigravida compared to multigravida. Further, the prevalence of malaria was higher in the first and second trimesters than the third trimester of pregnancy. The majority of infections were due to Pf (>87%). The malaria prevalence in our study (23.4%) was similar to a study conducted in other tribal belts of India (29.3%)31 but lower than that reported in central or west Africa (28.2%–41.9%) and comparable to that with East-Southern Africa (22.4%–36.5%) and other Southeast Asian countries (5%–37%).32
The high prevalence of malaria was seen during ANC follow-up in our study, but declined by 50% after delivery. Moreover, Pf parasitaemia was significantly higher at enrolment than after delivery. Pregnancy presents higher risk of malaria infection among women in this region as previous studies in the same area revealed 7.6%–17.1% prevalence of malaria among non-pregnant adults (aged >14 years).9,25
Community-based active surveillance revealed significantly higher burden of malaria than health centre-based passive surveillance in this region.33 Earlier hospital-based studies in Chhattisgarh and Jharkhand states revealed a malaria prevalence in the range of 1.3%–3.5% at antenatal clinics and 2.4%–3.6% using placental microscopy at the delivery unit.23,34,35 Whereas, active malaria case screening revealed a high prevalence ranging from 23.4% (our study) to 29.3% in forested areas of Chhattisgarh, Andhra Pradesh and Telangana.31
Fever, anaemia and splenomegaly are characteristic signs of malaria. In areas of stable malaria transmission, asymptomatic infections or afebrile parasitaemia is common among different age groups, including pregnant women (mainly due to placental sequestration of Pf) and is a major challenge for any control/elimination programme.25,32 In our study, 81.5% infections were asymptomatic at the time of primary screening. A high slide positivity rate of 29.2% among asymptomatic individuals from the same area has been reported earlier.25
Prevalence of anaemia in our study was similar to that observed in other parts of India and other endemic settings (60%–92%).23,31,34,35 Anaemia is widely prevalent in developing countries, particularly among pregnant women and severe anaemia has been found to be significantly associated with lower birth weight.36 Placental malaria may also increase the risk of maternal anaemia.37 Infections, metabolic deficiencies and genetic causes are the other main factors causing anaemia. Unlike earlier reports from different geographical areas, severe anaemia was significantly higher in our study (4%–10% v. 17.3%).23,34,38,39 However, anaemia and severe anaemia prevalence associated with Pf and Pf+Pv/Pm infections was relatively higher compared to Pv infections in our study although not statistically significant. In a hospital-based study done in western India, Pf malaria was found to be an important cause of the high mortality rate and severe anaemia among pregnant compared to non-pregnant women (37.8% v. 14.8% and 20% v. 4.1%, respectively).22,40
In our study, 33 of 350 pregnant women who were followed up had repeated malaria infection (85% due to Pf), which may lead to chronic infection. However, we could not ascertain whether the second infection was due to a new infection or persistence of previous infection due to inappropriate compliance of antimalarial drug. Clinically, this subset of pregnant women had a higher degree of severe anaemia (32%). During MIP, chronic infections have been closely associated with decreased birth weight due to foetal growth restriction and are associated with lower maternal haemoglobin and severe anaemia in endemic areas.30
Previous studies have also found that the malaria parasite (particularly Pf) is more likely to be present in placental smear than a mother’s peripheral smear and sometimes even after treatment.23,34,41,42 Placental histology is even superior to detect malaria infection than blood smears.37 However, we did not assess the burden of placental malaria during pregnancy. Malaria screening in our study was based on microscopy and RDT, which are considered less sensitive than polymerase chain reaction (PCR) to detect sub-microscopic asymptomatic malaria infections, but we could not do PCR.43 RDT test was helpful in detecting malaria infection and treatment of pregnant women in this remote and forested field area at the time of enrolment and follow-up.
The majority of pathological consequences of Pf during MIP are due to the sequestration of infected red blood cells to placental intervillous space via molecular interactions of VAR2 to placental chondroitin sulphate A (CSA) and associated inflammatory responses.44 Sequestration may result in hypoxia, anaemia and high fever. However, the exact pathogenesis (hypoxia, microvascular damage and angiogenic repair deficiencies mediated by VEGF, etc.) of placental malaria and associated adverse outcomes is still unknown.45 Recently, a placental malaria protein ‘VAR2CSA’ showed promise for novel cancer diagnostics and therapeutics.46 Thus, there is a need for molecular characterization of malaria parasites (Var 2 gene, especially from the placenta) from different endemic areas.
In our study, factors such as primigravida status, first and second trimester, women belonging to indigenous ethnic tribal groups and cash crop insufficiency (whole year) were independent risk factors for higher prevalence of malaria. Previous studies from eastern India revealed first and second pregnancies, fever within the past week and women belonging to rural areas were significant risk factors for higher prevalence of malaria among pregnant women.39 Similarly, a longitudinal cohort study in central India found that individuals belonging to ethnic tribal groups, lower economic strength of family (yearly income) and larger family size are also independent risk factors for higher malaria prevalence.47
Our study highlights high malaria prevalence, gravidity-wise trend of malaria and parasitaemia and risk factors for malaria among pregnant women in a remote and forested region of Madhya Pradesh. We also feel screening of asymptomatic carriers to be necessary for malaria control programmes. We found a high and perennial burden of malaria in pregnancy in this area, Anganwadi-based regular malaria screening using RDTs and complete treatment of pregnant women by an ANM or trained ASHA worker may be helpful in controlling malaria among pregnant women in this remote and forested region. Strengthening of health sub-centres operation in these areas may improve the malaria control programme’s effectiveness.
ACKNOWLEDGEMENTS
We are grateful to the Director, ICMR-NIRTH, Jabalpur for providing necessary facilities. We thank technical staff Mr Vijay Kacchi and Mr Ajesh Dubey for support during the field study. We are thankful to Dr Ravindra Chhabra for critical comments and suggestions to improve the final version of this manuscript. We are also thankful to Mr M.P. Singh for his valuable suggestions during data analysis. The manuscript has been approved by the Publication Screening Committee of ICMR-NIRTH, Jabalpur and assigned with the number ICMRNIRTH/PSC/18/2021.
References
- Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg. 1991;85:424-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody responses to Plasmodium falciparum and Plasmodium vivax blood-stage and sporozoite antigens in the postpartum period. Sci Rep. 2016;6:32159.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria and pregnancy in Cameroonian women. Effect of pregnancy on Plasmodium falciparum parasitemia and the response to chloroquine. Trop Med Parasitol. 1992;43:1-5.
- [Google Scholar]
- Quantifying the number of pregnancies at risk of malaria in 2007: A demographic study. PLoS Med. 2010;7:e1000221.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75-88.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of malaria in pregnancy as Latin America approaches elimination. Trends Parasitol. 2016;32:416-27.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a malaria rapid diagnostic test for assessing the burden of malaria during pregnancy. Am J Trop Med Hyg. 2004;70:481-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of Plasmodium falciparum malaria in pregnancy in subSaharan Africa: The challenges and public health implications. Parasitol Res. 2008;102:333-42.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of malaria in pregnancy in central India. Bull World Health Organ. 1999;77:567-72.
- [Google Scholar]
- The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1-2 Suppl):28-35.
- [CrossRef] [PubMed] [Google Scholar]
- Placental Plasmodium vivax infection and congenital malaria in central India. Ann Trop Med Parasitol. 2003;97:875-8.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374-81.
- [CrossRef] [PubMed] [Google Scholar]
- Anaemia-and malaria-attributable low birthweight in two populations in Papua New Guinea. Ann Hum Biol. 1997;24:547-55.
- [CrossRef] [PubMed] [Google Scholar]
- Gaps in the childhood malaria burden in Africa: Cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1-2 Suppl):57-67.
- [CrossRef] [PubMed] [Google Scholar]
- Changing scenario of malaria in central India, the replacement of Plasmodium vivax by Plasmodium falciparum (1986-2000) Trop Med Int Health. 2004;9:364-71.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of malaria among pregnant and non-pregnant women of district Jabalpur, Madhya Pradesh. Indian J Malariol. 1995;32:6-13.
- [Google Scholar]
- Malaria during pregnancy and its effects on foetus in a tribal area of Koraput District, Orissa. Indian J Malariol. 2000;37:11-17.
- [Google Scholar]
- Malaria during pregnancy and infancy, in an area of intense malaria transmission in central India. Ann Trop Med Parasitol. 2001;95:19-29.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of malaria in pregnancy in Jharkhand State, India. Malar J. 2009;8:210.
- [CrossRef] [PubMed] [Google Scholar]
- Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8:e73730.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of afebrile parasitaemia due to Plasmodium falciparum and P. vivax in district Balaghat (Madhya Pradesh): Implication for malaria control. Indian J Med Res. 2017;146:260-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid staining of malarial parasites by a water-soluble stain. Indian Med Gaz. 1944;79:102-4.
- [Google Scholar]
- Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25:452-7.
- [CrossRef] [PubMed] [Google Scholar]
- Studies on malaria during pregnancy in a tribal area of central India (Madhya Pradesh) Southeast Asian J Trop Med Public Health. 1998;29:10-17.
- [Google Scholar]
- High burden of malaria and anemia among tribal pregnant women in a chronic conflict corridor in India. Confl Health. 2017;11:10.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria in pregnancy. Mediterr J Hematol Infect Dis. 2013;5:e2013010.
- [CrossRef] [PubMed] [Google Scholar]
- Active v. passive surveillance for malaria in remote tribal belt of Central India: Implications for malaria elimination. Pathog Glob Health. 2016;110:178-84.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria prevalence among pregnant women in two districts with differing endemicity in Chhattisgarh, India. Malar J. 2012;11:274.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of two artemisinin-based combinations for the treatment of malaria in pregnancy in India: A randomized controlled trial. Malar J. 2018;17:246.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737-43.
- [CrossRef] [PubMed] [Google Scholar]
- Placental infections with histologically confirmed Plasmodium falciparum are associated with adverse birth outcomes in India: A cross-sectional study. Malar J. 2014;13:232.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria is an important cause of anaemia in primigravidae: Evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Malaria Infection and Risk Factors Associated with Anaemia among Pregnant Women in Semiurban Community of Hazaribag, Jharkhand, India. Biomed Res Int. 2015;2015:740512.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality trends in falciparum malaria-effect of gender difference and pregnancy. J Assoc Physicians India. 1999;47:774-8.
- [Google Scholar]
- The sick placenta-the role of malaria. Placenta. 2004;25:359-78.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a rapid diagnostic test for assessing the burden of malaria at delivery in India. Am J Trop Med Hyg. 2005;73:855-8.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of submicroscopic and asymptomatic malaria in India revealed from epidemiology studies at three varied transmission sites in India. Sci Rep. 2019;9:17095.
- [CrossRef] [PubMed] [Google Scholar]
- Placental malaria: A new insight into the pathophysiology. Front Med (Lausanne). 2017;4:117.
- [CrossRef] [PubMed] [Google Scholar]
- Placental hypoxia during placental malaria. J Infect Dis. 2008;197:757-65.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell. 2015;28:500-14.
- [CrossRef] [PubMed] [Google Scholar]
- Socioeconomic and household risk factors of malaria in tribal areas of Madhya Pradesh, central India. Indian J Med Res. 2015;141:567-75.
- [Google Scholar]




