Translate this page into:
Challenges during implementation of point-of-care testing in a multispecialty children’s hospital
Correspondence to SHIEFA SEQUEIRA; drshiefa31@yahoo.co.in
[To cite: Sequeira S, Lone R, Anjum S. Challenges during implementation of point-of-care testing in a multispecialty children’s hospital. Natl Med J India 2025;38:35–40. DOI: 10.25259/NMJI_659_2022.]
Abstract
Point-of-care testing (POCT) has been one of the fastest-growing areas of critical care management. It continues to increase in volume and complexity over the past few years and is now moving to the molecular level. POCT is currently defined as a testing process done near or at the site of patient care by non-laboratory clinical staff. Since these tests are done by clinical staff with minimum technical knowledge, many challenges arise due to a lack of understanding of the process of POCT.
The key challenges faced during the successful step-by-step implementation and execution of POCT at our multi-specialty hospital for children in the Middle East included POCT instrumentation, training and competency, quality control issues, proper handling of reagents and consumables, importance of critical call notification, patient identification errors, data management, etc.
Many clinical decisions are made based on the results of POCT, hence care should be taken during every step of the POCT process. If implemented appropriately, POCT can have a positive impact on operational efficiency and patient care. Healthcare organizations should innovate rapidly to meet the challenges of POCT.
INTRODUCTION
The rapid evolution of point-of-care testing (POCT) has led to the modernization of care of children. In the past decades we have seen a phenomenal growth in point-of-care laboratory testing and availability.1 This has enabled us to perform POCT tests on children of all ages, including premature infants. The primary aim of POCT is to intervene early. This is important in the acute care setting. The present guidelines state that blood equal to a maximum of 3% of the total body volume may be drawn per day from infants <2 months old, and no more than 10% of the total body volume may be drawn per day from infants >2 months of age.2 Hence, for children it is ideal to use POCT instruments that need only a single drop or small number of samples without any sample preparation such as whole blood, urine, or other body fluids for testing. Also, it is beneficial for patients who require multiple draws during their hospitalization (e.g. children with diabetes), those with difficult blood draws (e.g. premature infants), children especially those with cancers, those with blood loss, and those requiring quicker optimization of treatment such as anticoagulation, etc.
Paediatric POCT is broadly defined as a diagnostic test done on children, including preterm infants and newborns. The rapid turnaround time,3 ease of draw, the flexibility of the portable devices, simple operational procedures especially in critical situations, can speed up medical decision-making,4 thereby enhancing satisfaction of the family as children depend on their parents or adult caregivers for their care. However, POCT has disadvantages too. A wide and diverse range of geographically dispersed operators, instruments and stocks can increase the risk of errors and may require optimization to avoid these errors. Around 42% of tests in our hospital are performed on POCT instruments. We discuss the challenges encountered during the successful planning and execution of POCT at our multi-specialty free standing children’s hospital in the Middle East.
PLANNING–EXECUTION–MONITORING CYCLE
Planning of our POCT project was initiated in various medical units of our paediatric hospital. To overview the entire implementation process, a multidisciplinary task force was created. Nine steps were implemented and monitored. These included:
Step 1: Constitution of a POCT committee team
Step 2: Selecting instrumentation for paediatric mode
Step 3: Quality check requirements
Step 4: Inventory management
Step 5: Training and competency
Step 6: Connectivity and data handling
Step 7: Critical call notification
Step 8: Infrastructure and support
Step 9: Identification of specimens
Once implementation was completed, continuous monitoring of progress was undertaken. Patient identification error was the criteria used to monitor the progress.
CHALLENGES AND SOLUTIONS
Step 1: Constitution of a POCT committee team
As our tertiary care children’s hospital became operational, there was a need to build a POCT department. Most patients were children of different ages who needed quick clinical decisions and treatment. A management meeting was held, and the responsibility for POCT was assigned to the laboratory department under the direction of the Laboratory leader. To meet our objective, the formation of an effective POCT committee was the primary task. A multidisciplinary committee bringing together stakeholders from various departments was constituted (Fig. 1). The POCT team began to meet every month for the first 3 months to discuss and plan the implementation. We discussed the mandate of POCT, accreditation, the organizational structure of POCT, and its cost-effectiveness. We also sited appropriate tests, connectivity of POCT to the laboratory information system, availability of standard operating procedures (SOP) and user manuals to end users, selection and evaluation of instruments and tests, validation protocols, proficiency testing, quality assurance, audits, and assessment of training and competency. The POCT organizational structure helped to articulate the roles and responsibilities of all those involved in the POCT (Fig. 2).
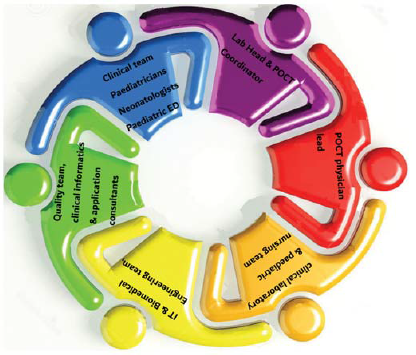
- Point-of-care testing (POCT) multispecialty committee at our paediatric hospital ED emergency department IT information technology
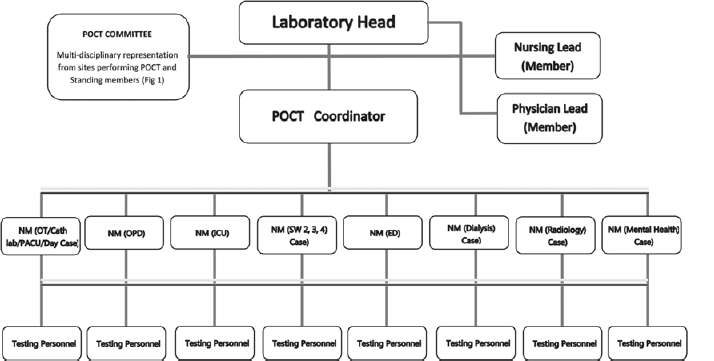
- Organizational chart for point-of-care testing (POCT) network at our paediatric hospital
The approval of the multidisciplinary committee was taken when crucial decisions had to be made. No decision was made without discussion at the POCT committee meeting. As the POCT service continued to grow, the POCT committee meetings became the convergence point for all major and minor issues on the POCT, including analytical and post-analytical issues.
Step 2: Criteria for selecting instrumentation in paediatric mode
In house validation by surveys and site visits. As we progressed, the second major challenge we encountered was selecting instruments for our paediatric patients. Numerous surveys and site visits were carried out, as we had to liaise with 15 paediatric services and 25 specialized clinics. The specialty clinics included:
Paediatric cardiology and cardiac surgery
Blood and cancer disorder clinic
Paediatric cleft and lip, paediatric dental clinic
Adolescent clinic
Paediatric diabetes
Paediatric dermatology
Paediatric endocrinology
Paediatric ENT
Paediatric gastroenterology
Paediatric mental health
Paediatric metabolic
Paediatric nephrology
Paediatric organ transplant centre
Paediatric neurology
Paediatric neurorehabilitation
Paediatric neurodevelopment and behavior
Paediatric orthopaedic
Paediatric plastic surgery
Paediatric pulmonology
Paediatric rheumatology
Paediatric sleep
Paediatric surgery
Paediatric urology
Services included paediatric outpatient department, emergency department, radiology, mental health, catheterization laboratory, dialysis unit, day surgery, postoperative unit, operation theatre, paediatric intensive care services, neonatal intensive care services, and the specialty wards.
Every department had its requirements; Cardiac and CATLAB required an ACT (activated clotting time), glucometer and blood gas instrument, the Pulmonology clinic needed sweat chloride test, the outpatients and emergency departments needed spot urine analysis and glucometer, Oncology needed urine analysis in order to check the specific gravity of urine during treatment, etc. During the instrument selection process, specific critical issues were addressed (Fig. 3).5 The overall impact of the POCT on management of patients was assessed. It is always essential to carry out an appropriate study to prevent problems after execution.6 The request for a new POCT was documented and discussed by the multidisciplinary committee.5
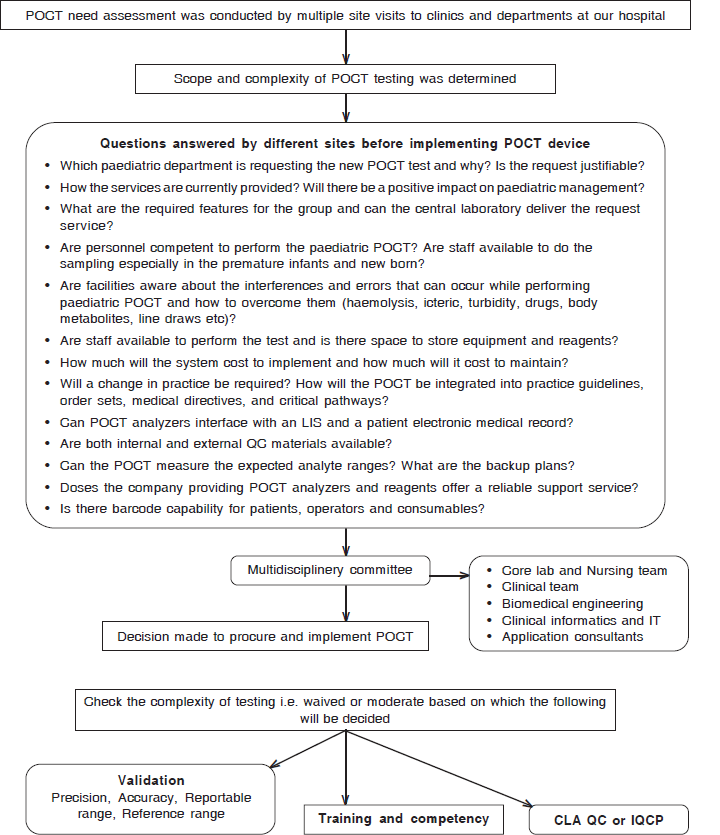
- Workflow for selection of a new point-of-care testing (POCT) instrument at our paediatric hospital LIS laboratory information service QC quality control
Performance evaluation criteria: The decision to implement the POCT was made after assessing the clinical need, cost comparisons, and analytical performance requirements. The performance of the POCT device was compared with that of the laboratory core analyzer.7 The limitations of the new devices were adequately studied and communicated to the clinicians who had to make clinical decisions based on the results obtained.8 Some of the things discussed were test sensitivity and specificity, the reportable range, possible interferences (drugs, diet, body metabolites, haemolysis), sample volumes, varying paediatric reference ranges at different development stages, different sample types (whole blood and serum/plasma) and dilution from line fluids.
In addition the instrument cost comparison and analysis also included both the visible and invisible cost. Visible cost included the reagent, cartridge, control, calibrator, reruns, proficiency testing, annual periodic preventive maintenance etc. Invisible costs include start-up/implementation, vendor involvement, equipment failures, unnecessary procedures, excessive inventory, time, etc.
Initial analytical performance requirements included a preliminary validation and evaluation of the instrument by assessing within and between day imprecision, method comparison using at least 20 patient samples with comparative laboratory (hospital/central laboratory), analytical measurement ranges (AMR) and linearity of the instruments. One of most important criteria for implementation was the method comparison and the results had to be within the acceptable tolerance limit. Other analytical performance requirements included evaluation of the reference ranges, flexibility of the instrument, patient safety, turnaround time, sample volume, consistency of results, quick service from the vendor, delivery of reagents and consumables on time, and disposal of instruments and reagents. In line with the above, a POCT needs assessment was conducted by meeting with various department members to gather information regarding the future practices and clinical needs for developing a POCT programme in our institute.
Step 3: Quality check, calibrations and proficiency requirements
Once the decision was made to move forward, the next step was to check the complexity of the testing, whether waived or moderate, based on which validation protocol, quality checks, calibration frequency and nature or frequency of competency and training were decided.6 SOPs were written for each test, and a copy was placed in the vicinity of each instrument.6,9 For the waived tests, we followed the manufacturer’s instructions for quality control. For moderately complex tests, a minimum of 2 levels of quality control were made mandatory each day before analysis of patient samples. For some instruments with an inbuilt simulator, e.g. ISTAT and Hemochron, we developed an individualized quality control plan (IQCP) after doing a proper risk analysis where the simulator was run daily, and external QC run once a month.
Calibration frequency depended on the nature of the test (waived/non-waived). For waived test manufacturer’s instructions were followed. For non-waived test calibration was performed following manufacturers instructions, at minimum which included the number, type and concentration of calibrator used, frequency and acceptable performance of the calibrator.
As we are a College of American Pathologists (CAP) accredited laboratory, proficiency testing (PT) surveys were selected for different individual POCT tests. PT surveys were also selected for inter-instrument comparison as we have many similar instruments in different locations. These samples were to be run three times a year. PT helps us to identify trends in results and correct mistakes if any.
Step 4: Inventory management
A further challenge was to manage reagents such as test strips, cartridges, quality control solutions, solution packs, and sensor cassettes, which is critical to achieving accurate patient outcomes. They are sensitive to temperature, moisture, and other storage conditions and may cause imprecise results.10 Meier and Jones,11,12 in their study of POCT errors, observed that improper handling of reagents was one of the three reasons for the latent condition in POCT, which could further amplify to a POCT error, the other latent condition being POCT operator incompetence, non-adherence of the operator to the SOP.
As POCT were performed by non-laboratory personnel whose primary focus was patient care, it was essential to acquaint them with the management of consumables, reagents, and QC materials used in the POCT process.13 Staff was trained to monitor changes in batch numbers, expiry dates, open stability, and follow manufacturer’s instructions. They were also trained to document room temperature, humidity, and refrigerator temperature logs on every 12-hour shift because fluctuations could alter the reagents. The SOPs were prepared and sent to end-users for pre-implementation review, including storage conditions, temperature, shelf life, etc.
Our most important finding during our monthly audit was the end-users failed to write the open stability dates on the reagents and QC after opening, which resulted in wastage of resources and wrong results. During troubleshooting of QC failures and questionable outcomes, available reagents with no expiry date were observed. There was a push to follow the guidelines and, again, training. Currently, vendors have begun to incorporate data management software into their instruments that provide reports on devices’ use and workload. Users may be tracked and trained if much wastage is observed. Barcode scanning functions allow operators to scan reagents and orders to verify the current batch number. If the reagents are out of date or invalid, the software has a system where the batches are locked and will no longer be accepted by the device. When new batches are used, data management provides alerts, identifying new batches and allowing batches to be verified.
Step 5: Training and competence
POCT at our hospital is generally done by trained clinical personnel such as paediatric nurses, perfusionists, respiratory therapists, radiology, and catheterization technicians who lack knowledge of different laboratory total testing phases. In addition to this, they are also skilled in providing expert care to children of all ages, including premature infants, simultaneously also working with the family to address their worries, anxieties, problems, and options. Even with sufficient training, the stresses of a hectic clinical environment might result in gaps or violations during the POCT process, giving less time and importance to particular tasks required for laboratory testing such as QC reviewing, temperature monitoring, machine maintenance, and documentation. Consequently, the results generated may be less accurate and precise and depend on the POCT operator’s proficiency level. Furthermore, the fact that clinical decisions are made instantaneously after a POCT result is obtained increases the risk of adverse events to the patient if the results are inaccurate and misleading. To resolve these problems, operators must regularly undergo training on the POCT device and check their competence.14
As our hospital is certified by CAP, we follow CAP guidelines for training and skills.15 For waived testing, e.g. training and competency assessment are conducted when a person joins the organization, and yearly subsequently. For non-waived tests, e.g. blood gas, glucometer, etc. training and competency assessment are done when the staff joins the organization; these are then assessed after 6 and 12 months and then annually. Hands-on training are provided by vendors or POCT-coordinator. The staff is then asked to demonstrate the running of samples. Competency is than assessed through visual inspection and blind testing of known values. All the six elements mentioned in the CAP POCT checklist are assessed but not limited to direct observation of patient testing (patient identification, preparation, collection, handling, processing, and testing), monitoring the documentation, interpretation, and reporting of critical results; review of QC, PT and maintenance records, proficiency testing and problem-solving skills. The greatest challenge faced by us during the implementation of POCT was the timely completion of training, education, and competency assessment of the POCT operators as they were on different shifts of the day; also, the busy schedule was a major hindrance. Monitoring and surveillance of competency files for all devices was done manually.
Step 6: Connectivity and data handling
The integration of POCT devices into our electronic medical record (EMR) system was another key challenge we faced when implementing POCT in our organization. At the time of integration, the results obtained on POCT devices did not reach the patient’s permanent medical records. It was common practice for staff to document the POCT outside the patient room, frequently writing information about the patient encounter on a paper and transferring to the electronic health record (EHR) later. This resulted in entry and treatment delays, causing errors in treatment. Some employees, under pressure, even forget to enter or lost the machine prints, resulting in permanent loss of data and inconvenience to the patient.
An execution plan for the POCT was developed. The POCT project strategy was developed in accordance with guidance provided by the accreditation bodies. The existing workflow from the control to disseminate the result, including the critical results, was also analyzed in detail. New result entry manuals, workflows, and charts were created. Having a single band with all the POC results in a section allowed the laboratory to add to a single section instead of searching around different navigators’ bands. This made patient identification, operator tracking, and reducing the many complexities of the data flow more manageable. This data stream’s impact has resulted in better information management at the point of care and the integration of test results at the point of care into patient records.
Connectivity, integration, and data management are effective methods of capturing real-time patient outcomes, thereby delivering timely, accurate information to all patient care providers, thus increasing accuracy, patient safety, and eliminating billing issues.16,17 All results arrive at the patient’s permanent medical records and can be reviewed whenever needed. It eliminates the need to enter results by hand, reducing staff workload and transcription errors. Proper data management system enables the physician to order the test correctly, ensures the capture and documentation of all patient results into patient’s permanent records, automates billing, and reduces transcriptional errors, thus improving quality and safety.18 It also helps to correctly identify the patient and monitor the operator.19 All results records must be correctly identified and distinct from those carried out at the central laboratory. Otherwise, they will be deceptive to the referring physician and have the same CPT codes confusing billing. Thus it is recommended that there be an appropriate data management system for all POCT devices.
Step 7: Critical call notification
Handling and reporting critical results is one of the core areas of staff working in the central laboratory, but not of staff working in the POCT areas. Critical call notices must follow the same procedure as in the central laboratory. Reporting critical calls in a timely manner by POCT staff can prevent potential harm to the patient and speed up treatment. End users should be familiar with these critical values and how to alert clinicians. Critical notice is one of the POCT key performance indicators at our set-up. At the end of the month, the data is analyzed, and emails are sent to individuals who did not log critical calls. If needed, a refresher is given.
Step 8: Infrastructure and support
Akin to the central laboratory, POCT also requires proper infrastructure and resources to ensure quality reporting. Some of the concerns discussed in the meetings were dedicated space and area needed to accommodate testing. In situations where the instruments need to be moved, mobile carts were used which contained the supplies and the equipment. Disposal of POCT waste was another concern. Separate yellow bags and sharp containers were installed as required. Data monitoring softwares were used remotely to monitor changing test performances, e.g. Aqure software for blood gas, HD solutions for I-STAT. There were operated remotely. Issues of information technology were sorted as some worked with WiFi and others needed dedicated ports.
Step 9: Identification of specimens
Incorrect or missing identification was another major challenge for POCT. Staff often forgot to place the correct patient ID during sample processing. This is the most critical error in POCT. Incorrect identification can sometimes result in erroneous results in inpatient records, especially when POCT devices are bidirectional. Misidentification can result in incorrect treatment, misdiagnosis, compliance issues with local organizations and accreditation, loss of income, and recollection of the sample, causing profound malaise in patients. Some of the amplifiers for identification errors were transcriptional errors while manually labelling the specimen tubes, not following the two-patient identifier process, lack of proper workflow, and stress.20,21
One of our most notable accomplishments was reducing ABG errors over a year (Fig. 4), in our ICU. Notable errors were missing patient ID, missing sample type (capillary, arterial, venous), staff using their ID instead of patient ID, and invalid ID (Fig. 4). This was an effective communication effort among POCT committee members who reviewed the information in their respective departments.
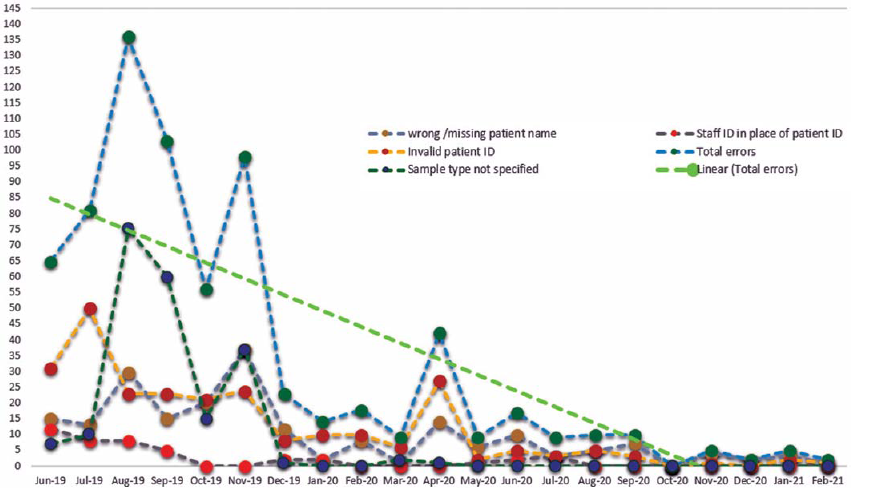
- Reduction of errors in the intensive care unit after proper implementation of point-of-care testing
Staff should be adequately trained to use at least two patient identifiers while collecting the samples, such as date of birth, name, accession number, etc. Where possible, the specimen tube, personnel, and patient identifiers must be analyzed at the patient’s bedside. Patient barcode labels must always be used to enter a patient ID in the analyzer prior to the run.
DISCUSSION
Prevention of medical errors has become a focus for enhancing the quality of healthcare in children. The key advantage of using POCT in a paediatric setting is to obtain quick results in order to intervene early and provide the best care. However, some limitations, such as accuracy, quality, and safety, must be borne in mind. These might pose challenges to the successful implementation of POCT. When implemented appropriately and thoughtfully, POCT can positively impact operational effectiveness and patient care.
Conflicts of interest
None declared
References
- Point-of-care diagnostics: Recent advances and trends. Biosensors (Basel). 2017;7:62.
- [CrossRef] [PubMed] [Google Scholar]
- Paediatric laboratory medicine. In: Contemporary practice in clinical chemistry (4th ed). London: Academic Press; 2016. p. :763-8.
- [Google Scholar]
- Implementation of point-of-care testing in a pediatric healthcare setting. Crit Rev Clin Lab Sci. 2019;56:239-46.
- [CrossRef] [PubMed] [Google Scholar]
- Practical challenges related to point of care testing. Pract Lab Med. 2016;4:22-9.
- [CrossRef] [PubMed] [Google Scholar]
- POCT 04-essential tools for implementation and management of a point-of-care testing program (3rd ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
- [Google Scholar]
- AACC guidance document on management of point-of-care testing. J Appl Lab Med. 2020;5:762-87.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for point-of-care testing: Haematology. Br J Haematol. 2008;142:904-15.
- [CrossRef] [PubMed] [Google Scholar]
- Leveraging the real value of laboratory medicine with the value proposition. Clin Chim Acta. 2016;462:183-6.
- [CrossRef] [PubMed] [Google Scholar]
- Best practices in the implementation of a point of care testing program: Experience from a tertiary care hospital in a developing country. EJIFCC. 2019;30:288-302.
- [Google Scholar]
- Patient safety in point-of-care testing. Clin Lab Med. 2004;24:997-1022.
- [CrossRef] [PubMed] [Google Scholar]
- Point-of-care testing error: Sources and amplifiers, taxonomy, prevention strategies, and detection monitors. Arch Pathol Lab Med. 2005;129:1262-67.
- [CrossRef] [PubMed] [Google Scholar]
- Point-of-care testing curriculum and accreditation for public health-enabling preparedness, response, and higher standards of care at points of need. Front Public Health. 2019;6:385.
- [CrossRef] [PubMed] [Google Scholar]
- Point-of-care testing (POCT): Current techniques and future perspectives. Trends Analyt Chem. 2011;30:887-98.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers to hospital-based clinical adoption of point-of-care testing (POCT): A systematic narrative review. Crit Rev Clin Lab Sci. 2016;53:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Organizational challenges in the management of point-of-care diagnostics in healthcare facilities. J Lab Med. 2020;44:103-5.
- [CrossRef] [Google Scholar]
- Managing the challenges in point-of-care testing--An ecosystem approach. Point of Care: J Near-Patient Testing Tech. 2013;12:76-9.
- [CrossRef] [Google Scholar]
- Management of a point-of-care testing program. Clin Lab Med. 2009;29:433-48.
- [CrossRef] [PubMed] [Google Scholar]
- Reducing medical errors through barcoding at the point of care. Clin Leadersh Manag Rev. 2004;18:328-34.
- [Google Scholar]
- Reducing patient identification errors related to glucose point-of-care testing. J Pathol Inform. 2011;2:22.
- [CrossRef] [PubMed] [Google Scholar]




